Veer Patel1*, Rakesh Patel2, Hetansh Shah1, Shyam Purohit1, Mayur Pawar1 and Abuzar Pathan1
1Faculty of Pharmacy, Department of Bachelor of Pharmacy (B. Pharm), Parul Institute of Pharmacy, Parul University, Vadodara, Gujarat, India
2Faculty of Pharmacy, Department of Pharmaceutics, Parul Institute of Pharmacy, Parul University, Vadodara, Gujarat, India
*Corresponding Author: Veer Patel, Bachelor of Pharmacy (B. Pharm), Parul Institute of Pharmacy, Parul University, Vadodara, Gujarat, India.
Received: March 02, 2021; Published: March 20, 2021
Background: Azithromycin is a poorly water soluble drug having low solubility. Dissolution is the rate-limiting step in absorption of such drugs. Therefore, solubility of such drugs need to be enhanced in order to have a stable and effective dosage form having greater bioavailability. Solid dispersion technique is one of the effective methods to enhance the solubility of poorly soluble drugs.
Aim and Objectives: The present study aimed at enhancing the solubility of biopharmaceutical classification system Class II Drug, i.e. Azithromycin using Mannitol and β-Cyclodextrin as a carrier.
Method: Solid dispersions of Azithromycin with Mannitol and β-Cyclodextrin were prepared by Melting, Kneading and Solvent Evaporation method. The solubility of these prepared dispersions was evaluated.
Results: Solubility of prepared dispersions of Azithromycin were reported in µg/mL. The solubility of solid dispersion which was prepared using the Drug: Mannitol mixture in 1:4 ratio was found to be maximum, i.e. reported solubility of 7.8 µg/mL. The solubility of solid dispersion which was prepared using Drug: β-Cyclodextrin mixture in 1:1.5 ratio was found to be maximum, i.e. reported solubility of 9.52 µg/mL. The solubility of those dispersions that were prepared using Melting and Kneading Method were found to be maximum.
Conclusion: Drug having less aqueous solubility can have an enhanced rate of dissolution by using solid dispersion technique.
Keywords: Azithromycin; Solubility; Solid Dispersion; BCS (Biopharmaceutical Classification System); Absorbance
BCS: Biopharmaceutical Classification System; HCL: Hydrochloric Acid.
Azithromycin, a macrolide antibiotic of the azalide subclass, exerts its antibacterial action by binding to the 50s ribosomal subunits of susceptible bacteria and suppressing protein synthesis; however, it differs chemically from erythromycin in that a methyl-substituted nitrogen atom is incorporated into the lactone ring. Azithromycin is a white crystalline powder with a molecular formula of C38H72N2O12 and a molecular weight of 749.0 gm/mol. Azithromycin is a broad spectrum antimicrobial agent with oral bioavailability about 37%. The drug has pKa values around 8.74 and is sparingly soluble in water (~ 2.37 mg/L) and soluble in ethanol [1,2].
| Sr. No. | BCS Class | Solubility | Permeability | Example |
|---|---|---|---|---|
1 |
Class I |
High |
High |
Metoprolol, Verapamil |
2 |
Class II |
Low |
High |
Gilbenclamide, Phenytoin |
3 |
Class III |
High |
Low |
Cimetidine, Captopril |
4 |
Class IV |
Low |
Low |
Cholorthiazide, Taxol |
Table 1: The Biopharmaceutical Classification System.
The Biopharmaceutical Classification System (BCS) has been developed to provide a scientific approach for classification of drugs based on solubility in relation to dose and the permeability in combination with the dissolution properties of the dosage form [3]. According to the BCS, Azithromycin can be classified as a Class II drug; therefore the drug dissolution may be the rate-limiting step in the drug absorption process. It shows erratic dissolution problem in gastric and intestinal fluid due to its poor water solubility. Rate of absorption and/or extent of bioavailability for such insoluble drugs are controlled by rate of dissolution in gastrointestinal fluids. The effort to improve the dissolution and solubility of a poorly water-soluble drug remains one of the most challenging tasks in drug development. Several methods have been introduced to overcome this problem like micronization, solubilization, salt formation, complexation with polymers, change in physical form, solid dispersions, complexation and the use of hydrophilic carriers [1,4].
Solid dispersionsThe term solid dispersion refers to a group of solid products consisting of at least two different components, generally a hydrophilic matrix and a hydrophobic drug. The matrix can be either crystalline or amorphous. The drug can be dispersed molecularly, in amorphous particles (clusters) or in crystalline particles. Transformation of crystalline drug to amorphous drug upon solid dispersion formulation increases the dissolution rate. Solid dispersion techniques have been used to increase the solubility of a poorly water soluble drug. Solid dispersion is a viable and economic method to enhance bioavailability of poorly water soluble drug [4,5].
Methods of preparing solid dispersionsVarious methods for preparation of Solid Dispersions are as shown in the figure 1.
Figure 1: Types of methods for preparation of Solid Dispersion.
In this method, carrier is permeated with water and transformed to paste. Drug is then added and kneaded for particular time. The kneaded mixture is then dried and passed through sieve if necessary.
AdvantagesIn this method, both drug and carrier are dissolved in organic solvent. After entire dissolution, the solvent is evaporated. The evaporated film is formed which is then removed using spatula for getting a powder mixture.
AdvantagesRequired amount of drug is added to the solution of carrier. The system is kept under magnetic agitation and protected from the light. The formed precipitate is separated by vacuum filtration and dried at room temperature in order to avoid the loss of water from the inclusion complex.
Melting methodDrug and carrier are mixed using mortar and pestle. To accomplish a homogenous dispersion the mixture is heated at or above the melting point of all the components. It is then cooled to acquire a congealed mass. It is crushed and sieved.
AdvantagesPhysical mixture of drug and carrier is mixed for some time employing a blender at a particular speed. The mixture is then charged into the chamber of a vibration ball mill steel balls are added. The powder mixture is pulverized. Then the sample is collected and kept at room temperature in a screw capped glass vial until use.
Gel entrapment techniqueHydroxyl propyl methyl cellulose is dissolved in organic solvent to form a clear and transparent gel. Then drug for example is dissolved in gel by sonication for few minutes. Organic solvent is evaporated under vacuum. Solid dispersions are reduced in size by mortar and sieved.
Spray-drying methodDrug is dissolved in suitable solvent and the required amount of carrier is dissolved in water. Solutions are then mixed by sonication or other suitable method to produce a clear solution, which is then spray dried using spray dryer.
Lyophilization techniqueFreeze-drying involves transfer of heat and mass to and from the product under preparation. This technique was proposed as an alternative method to solvent evaporation. Lyophilization has been thought of a molecular mixing technique where the drug and carrier are co dissolved in a common solvent, frozen and sublimed to obtain a lyophilized molecular dispersion [6].
This study aimed at enhancing solubility of BCS Class II Drug, i.e. Azithromycin. In present study attempts were made to enhance the dissolution of Azithromycin using Solid Dispersion technique. Solid Dispersions of Azithromycin with Mannitol and β-Cyclodextrin and using appropriate solvent, i.e. Chloroform. The dispersions were prepared in different ratios using the Melting method, Kneading method and Solvent Evaporation method and those prepared formulations were evaluated. The most suitable formulation having maximum enhanced solubility was examined and the most suitable method of preparation from the selected methods of preparing the solid dispersions was evaluated and inferred.
Azithromycin was obtained as gift sample from Chemdyes Corporation, Rajkot, Gujarat. Mannitol (Suvidhinath), β-Cyclodextrin (Himedia Laboratories), Chloroform (Loba Chemi Pvt. Ltd.), Hydrochloric Acid (Fischer Scientific), Sodium Hydroxide (Atur Instru Chem), Potassium Dihydrogen Phosphate (Chemdyes) were provided by Parul Institute of Pharmacy and were of pharmaceutical and analytical Grade. Instruments like Weighing Balance (Scale Tec), UV Spectrophotometer (Shimadzu – 1700), Melting Point Apparatus (AnuLab) and Tray Dryer (Das Lab Instrument) were utilized for conducting the research work.
Methods Characterization and identification of drug Physical characterization of drugThe drug was evaluated for physical characteristics. The drug was kept on a clean surface and observed by naked eye and the observations were noted down.
Determination of melting pointThe surface morphological examinations of SLNs were carried out with scanning electron microscopy (Model JSM 5610 LV SEM, Japan).
Laser diffraction particle size analyzer (Zetasizer Nano S90, Malvern, UK) were used for determination of the particle size as well as particle size distribution of the SLNs.
Preparation of calibration curve Preparation of standard stock solution and working standard solution10 mg of Azithromycin was accurately weighed and transferred to 10 mL of volumetric flask and volume was made up with 0.1 N HCl (Hydrochloric Acid) to get standard stock solution of concentration 1000 µg/mL.
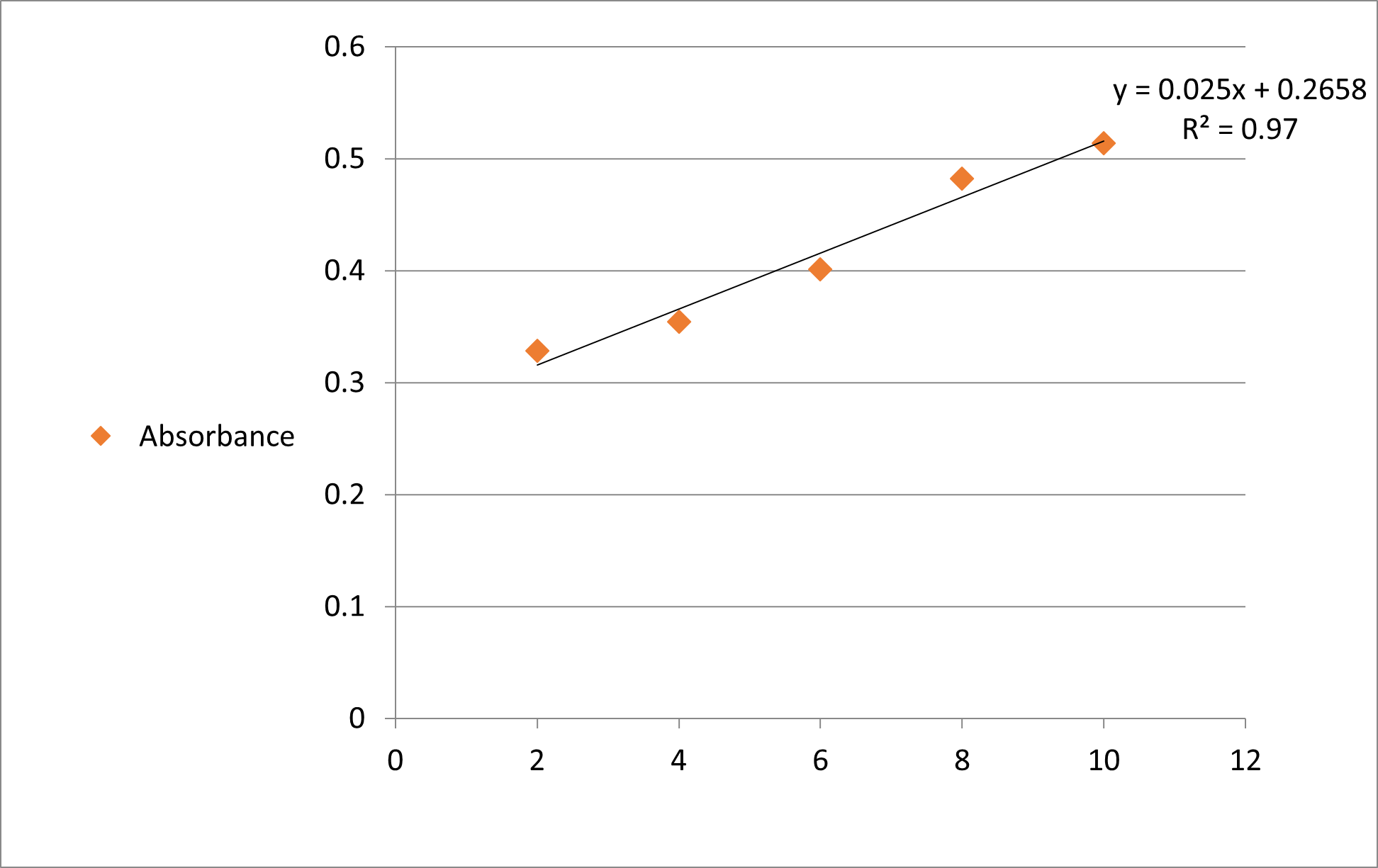
Figure 2: Calibration Curve.
The solid dispersions of Azithromycin and Mannitol (carrier) in various drug-to-carrier weight ratios were prepared by melting method, kneading method and solvent evaporation method. Similarly solid dispersions of Azithromycin and β-Cyclodextrin (carrier) in various drug-to-carrier weight ratios (similar as for Mannitol) were prepared by melting method, kneading method and solvent evaporation method.
| Sr. No. | Azithromycin | Mannitol | Drug: Carrier Ratio |
|---|---|---|---|
1. |
100 mg |
100 mg |
1: 1 |
2. |
100 mg |
200 mg |
1 2 |
3. |
100 mg |
300 mg |
1: 3 |
4. |
100 mg |
400 mg |
1: 4 |
5. |
100 mg |
500 mg |
1: 5 |
Table 2: Formulation Composition of Azithromycin: Mannitol.
| Sr. No. | Azithromycin | β-Cyclodextrin | Drug: Carrier Ratio |
|---|---|---|---|
1. |
100 mg |
50 mg |
1: 0.5 |
2. |
100 mg |
100 mg |
1: 1 |
3. |
100 mg |
150 mg |
1: 1.5 |
Table 3: Formulation Composition of Azithromycin: β-Cyclodextrin.
For Qualitative identification of Drug, 25 mg of Azithromycin was weighed and added into 25 mL of Volumetric Flask and the volume was made up to the mark with distilled water.
Solubility of powdered solid dispersions25 mg of powdered dispersion (obtained by all the three methods) was weighed and added into 25 mL of Volumetric Flask and the volume was made up to the mark with distilled water. The solution mixture was then filtered and the absorbance was taken. The solubility was then calculated using the calibration curve.
Following were the observations on physical identification of the drug which are:
| Name of the Drug | Observed Melting Point | Standard Melting Point |
Azithromycin |
110 oC - 116 oC |
113 oC - 115 oC |
Table 4: Observation for Determination of Melting Point.
Standard curve for the estimation was prepared in phosphate buffer pH 6.8 in concentration range of 2-10 μg/ml. In this concentration range good linearity was observed with the correlation coefficient (R2) 0.97. The absorbance of pure drug was found to be 0.408 nm.
Absorbance of prepared dispersions| Method of Preparation | Absorbance of Azithromycin: Mannitol (nm) | ||||
|---|---|---|---|---|---|
| 1: 1 | 1: 2 | 1: 3 | 1: 4 | 1: 5 | |
Kneading Method |
0.104 |
0.124 |
0.272 |
0.320 |
0.268 |
Melting Method |
0.108 |
0.157 |
0.268 |
0.460 |
0.391 |
Solvent Evaporation Method |
0.137 |
0.186 |
0.270 |
0.348 |
0.332 |
Table 5: Absorbance of Solid Dispersion of Azithromycin: Mannitol.
| Method of Preparation | Absorbance of Azithromycin: β-Cyclodextrin (nm) | ||
|---|---|---|---|
| 1: 0.5 | 1: 1.0 | 1: 1.5 | |
Kneading Method |
0.365 |
0.425 |
0.503 |
Melting Method |
0.279 |
0.449 |
0.478 |
Solvent Evaporation Method |
0.280 |
0.344 |
0.362 |
Table 6: Absorbance of Solid Dispersions of Azithromycin: β-Cyclodextrin.
From the obtained standard linear equation for calibration curve, i.e. y = 0.025x + 0.02658 (y = mx + c), using the noted observations of absorbance for individual Drug: Carrier Dispersions, following solubility for both the type of dispersion mixtures were reported.
| Method of Preparation | Solubility of Azithromycin: Mannitol (µg/mL) | ||||
|---|---|---|---|---|---|
| 1: 1 | 1: 2 | 1: 3 | 1: 4 | 1: 5 | |
Kneading Method |
-6.44 |
-5.64 |
0.28 |
2.2 |
0.12 |
Melting Method |
-6.28 |
-4.32 |
0.12 |
7.8 |
5.04 |
Solvent Evaporation Method |
-5.12 |
-3.16 |
0.2 |
5.72 |
2.79 |
Table 7: Solubility of Solid Dispersion of Azithromycin: Mannitol.
| Method of Preparation | Solubility of Azithromycin: β-Cyclodextrin (µg/mL) | ||
|---|---|---|---|
| 1: 0.5 | 1: 1.0 | 1: 1.5 | |
Kneading Method |
4.0 |
6.4 |
9.52 |
Melting Method |
0.56 |
7.36 |
8.52 |
Solvent Evaporation Method |
0.6 |
3.16 |
3.8 |
Table 8: Solubility of Solid Dispersions of Azithromycin: β-Cyclodextrin.
The solubility of Azithromycin: Mannitol dispersion was found to be maximum (7.8 µg/mL) in the Drug: Carrier ratio of 1:4 which was prepared utilizing the melting method. Likewise, the solubility of Azithromycin:β-Cyclodextrin was found to be maximum (9.52 µg/mL) in the Drug: β-Cyclodextrin ratio of 1:1.5 which was prepared utilizing the kneading method.
Solid Dispersions is one of the methods utilized for enhancing the solubility of poorly soluble drugs. For poorly aqueous soluble drugs, solid dispersion method is proved to be effective for establishing dissolution enhancement. In present study, Azithromycin – a BCS Class II drug was identified using physical characterization and melting point determination. The solid dispersions of Azithromycin were prepared using melting, kneading and solvent evaporation method. The absorbance of prepared dispersions were reported using UV Spectrophotometer and solubility of individual Drug: Carrier mixture was reported in µg/mL. The solubility of Azithromycin: Mannitol mixture (7.8 µg/mL) and Azithromycin:β-Cyclodextrin mixture (9.52 µg/mL) was found to be maximum. Both these dispersions having maximum solubility were prepared by using melting method and kneading method. From the observations of the study, it can be concluded that melting method and kneading method were effective in enhancing the solubility of the prepared solid dispersions of Azithromycin. Thus, solubility of poorly soluble drugs can be enhanced by using solid dispersion technique.
The authors are thankful to Dr. Abhay Dharamsi – Principal, Parul Institute of Pharmacy and Dean, Faculty of Pharmacy, Parul University for his motivation and support in completing the entire study. The authors are also thankful to Parul Institute of Pharmacy for providing the gift sample and for providing all the requirements and equipment’s for conducting the study.
The authors declare that there are no potential conflicts of interest in relation with this article.
Citation: Veer Patel., et al. “Solubility Enhancement of Azithromycin by Solid Dispersion Technique Using Mannitol and β-Cyclodextrin". Acta Scientific Pharmaceutical Sciences 5.4 (2021): 48-54.
Copyright: © 2021 Veer Patel., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.