Homady MH1*, Juma ASM1, Ubeid MH2, Salih TS1 and Al-Jubori MM3
1Department of Biomedical Sciences, College of Science, Cihan University-Erbil, Kurdistan, Iraq
2Department of General Biology, College of Science, Cihan University-Erbil, Kurdistan, Iraq
3College of Medicine, Babylon University, Iraq
*Corresponding Author:Homady MH, Department of Biomedical Sciences, College of Science, Cihan University-Erbil, Kurdistan, Iraq.
Received: February 08, 2021; Published: February 27, 2021
Colorectal cancer (CRC), which is also referred to colorectal adenocarcinoma, occurs when the growth of cells goes out of control in the colon or rectum. A number of histological colorectal carcinoma are listed, such as mucinous, signet ring cell, and moderately differentiated adenocarcinoma. The present study included fifty tissue blocks (16 females and 34 males) of patient groups with CRC and thirty five tissue blocks of colon tissue (ulcerative colitis) which were used as control group. The mean age of patients group was 51.44 ± 16.67 years. The majority of patients with colonic carcinoma were above the age of 40, accounting for 80%, while 20% of cases were below the age of 40 years. A recto-sigmoid location is the most common site for colonic tumors accounting for 60%. Grade of tumor was well differentiated in 56%, and the following features were observed: The tissue appears with multi-layering, back to back arrangement (little intervening stroma), loss of polarity, loss of goblet cells, and invasion of stroma and presence of nuclear criteria of malignancy: hyperchromatism, high N/C ratio visible nucleoli and abnormal mitosis. The present results also showed that in grade I lesion, most of tumor retains glandular pattern, moderately differentiated in 28%, and tumor is nearly equally composed of glandular and solid patterns. However, the poorly differentiated was 16% with same cellular criteria of malignancy but almost all the tumor was composed of solid areas. The present findings divided the stage of tumor patients into: 22% stage I; 66% stage II, and 12% stage III.
Keywords: Colorectal; Age; Gender; Carcinoma; Malignancy
Colorectal cancer (CRC) is the 2nd most common cancer in females and the 3rd most common cancer in males [1]. This disease is the most common malignancy in men with 75 years of age and over. It has been concluded that over one million people develop CRC annually, where the disease specific mortality rate being in the developed world [2].
Lifestyle, genetic and environmental factors were found to be of some of the factors that make CRC a multifactorial disease. Even though CRC could be hereditary and non-hereditary, however, the non-hereditary type is the most common and mainly caused by somatic mutations in response to environmental factors. Colorectal tumours appear with a wide variety of abnormal tissue growths (malignant tumours) ranging from benign tumours to infiltrating cancer and are primarily tumours that developed from epithelial cells (namely, adenomas or adenocarcinomas). Genetic change in the epithelial cells of colon is considered the essential process in the etiology of colorectal carcinoma. However, liver and lung distant metastases in CRC are common [3]. The peak incidence of CRC is at the age of 60 to 70 years and fewer than 20% of cases occur before age of 50, males being slightly more affected than females. Most CRC occurs sporadically, where 25% of the patients were found to have a family history of the disease, suggesting that shared genes and environment may contribute to the disease [4].
Rates of CRC increase with environmental factors that may represent risk factors [5].
Diets high in total fat and meat, both red and white meats, appear to be associated with developing adenomatous polyps and an increased incidence of CRC risk [6,7]. The use of some drugs and supplements, non-steroidal anti-inflammatory diseases (NSAIDs), estrogens, folic acid, and calcium might prevent the development of CRC [8,9]. The CRC under the various types of adenocarcinoma is: mucinous coiled adenocarcinoma (> 50 mucinous), small-cell (oat cell) carcinoma, squamous cell carcinoma, signet-ring carcinoma, adenosquamous carcinoma, medullary carcinoma, and undifferentiated carcinoma [10]. A malignant epithelial tumor is the most common adenocarcinoma, originating from glandular epithelium of the colorectal mucosa. CRC are adenocarcinoma originating from epithelial cells of the colorectal mucosa and represent over 90% [11]. Glandular formation is what characterizes conventional adenocarcinoma, which is the basis for histological tumor grading. More than 95% of the tumor is gland forming in well differentiated adenocarcinoma. 50 - 95% of moderately differentiated adenocarcinoma shows gland formation. Poorly differentiated adenocarcinoma is mostly solid with less than 50% gland formation [12,13]. Large glandular structure with pools of extra cellular mucin is typical of mucinous adenocarcinoma. Many mucinous adenocarcinomas occur in patients with hereditary non polyposis CRC (HNPC or lynch syndrome) [14]. A prominent intra cytoplasmic mucin vacuole that pushes the nucleus to the periphery is characteristic of signet ring cell [15-17].
The incidence of CRC rates are the highest in Africa and Americans [18].
In addition to the overall mortality [19] when compared to white male and female patients. Etiologic factors could be the cause of these differences, such as smoking or diabetes mellitus [20]. The overall life time risk of CRC for men and women is similar numerically, even though most studies have shown an increased risk for men regarding advanced colorectal neoplasia as well as CRC [21,22].
The American Cancer Society [23] concluded that a person can develop CRC at any age, the risk being increased greatly with age. In fact, more than 90% of colorectal cases are diagnosed in patients over the age of 50.
The aim of the present study was to clarify the relation of age and gender.
The present study was conducted in the laboratories of Molecular Biology, Faculty of Science, Kufa University and Al-Sadar Teaching Hospital in Al-Najef province. Fifty tissue blocks embedded in paraffin wax of colorectal cancer (CRC) (16 female and 34 males) were obtained as patients group. Thirty five other tissue blocks also embedded in paraffin wax from colon (ulcerative colitis) were collected randomly during the collection of malignant samples age and sex being matched, used as a control group. Five µm-thick sections were obtained from paraffin embedded tissues. These sections were processed and stained by using Haematoxylin and Eosin, the method described by [24]. Digital analysis was performed using image J software, and data were analyzed using two software programs, (SPSS) version 16 and Microsoft Office Excel (2010). For purpose of presentation, numeric variables were expressed in the form of mean ± SD (standard deviation). Mean values were compared using independent samples t-test. Chi-square test was used to study association between any two categorical variables.
In the present study, the mean age of patients was 51.44 ± 16.67 years and the median was 52 years, whereas the age range in patients group was from 21 years through to 85 years. The mean age of control group was 45.86 ± 15.73 and median age was 49 years, whereas the age range in control group was from 10 to 65 years.
The majority of patients with colonic carcinoma were more than 40 years of age, accounting for 80%, while 20% of cases were below the age of 40, fifteen cases out of 50 (30%) were between the age of 50 - 59 years (Table 1 and figure 1).
| Control | Colonic Carcinoma | |||
|---|---|---|---|---|
| Age Interval (years) | No. | % | No. | % |
10 - 19 |
3 |
8.57 |
0 |
0.00 |
20 - 29 |
3 |
8.57 |
7 |
14.00 |
30 - 39 |
3 |
8.57 |
3 |
6.00 |
40 - 49 |
9 |
25.71 |
11 |
22.00 |
50 - 59 |
6 |
17.14 |
15 |
30.00 |
60 - 69 |
11 |
31.43 |
6 |
12.00 |
70 - 79 |
0 |
0.00 |
5 |
10.00 |
≥ 80 |
0 |
0.00 |
3 |
6.00 |
Total |
35 |
100.00 |
50 |
100.00 |
Mean ± SD |
45.86 ± 15.73 years |
51.44 ± 16.67 years |
||
Table 1: Age distribution of patients with colonic carcinoma and control subjects (10 year intervals).
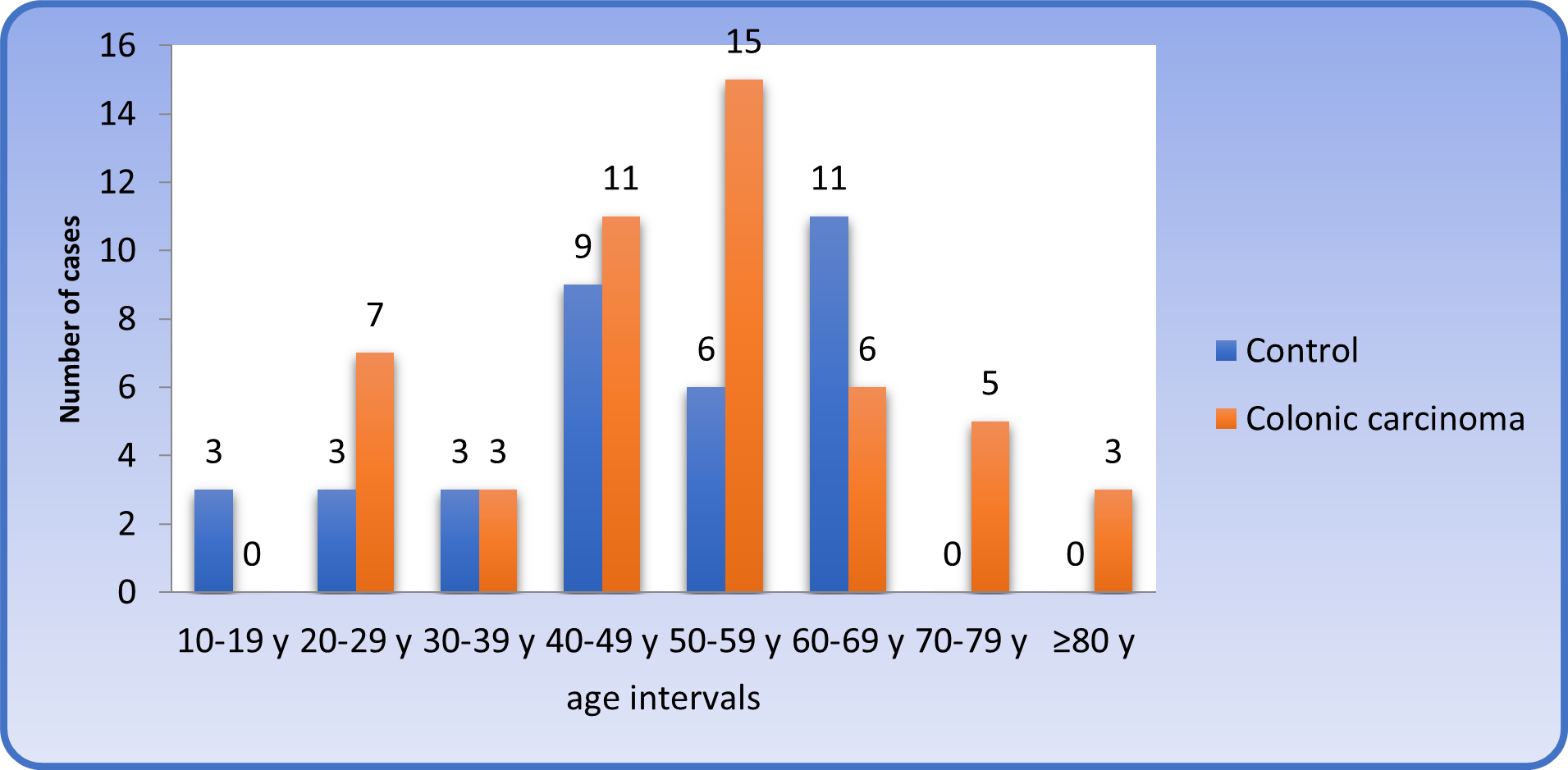
Figure 1: : Distribution of patients and control subjects (10 years intervals).
Gender of patients and control groupsThe patients’ group included 34 (68%) males and 16 (32%) females, while control group included 21 (60%) males and 14 (40%) females. Despite these minor differences in gender ratio between patients and control groups, there was no statistical difference.
The ratios of males to females in the present study were 1.5:1 and 2:1 in both control and patients groups, respectively. The mean age of male patients was 50.68 ± 17.48 years, whereas the mean age of female patients was 53.06 ± 15.22 years and there was no statistical difference in the mean age between male and female patients.
The mean age of male control subjects was 45.33 ± 16.97 years, while the mean age of females control subjects was 46.64 ± 14.23 years, which did not reveal any significant difference in the mean age between males and females control subjects. Performing a classification of patients by gender and age intervals revealed that most of the male patients were in the age interval of 50-59 years (32.35%). On the other hand, the female patients revealed the higher frequency in the ages between 50-59 years (25%), as shown in table 2.
| Control | Colonic Carcinoma |
|||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||
| Age Intervals (years) | No. | % | No. | % | No. | % | No. | % |
10 - 19 |
2 |
9.52 |
1 |
7.14 |
0 |
0.00 |
0 |
0.00 |
20 - 29 |
2 |
9.52 |
1 |
7.14 |
6 |
17.65 |
1 |
6.25 |
30 - 39 |
2 |
9.52 |
1 |
7.14 |
1 |
2.94 |
2 |
12.50 |
40 - 49 |
4 |
19.05 |
5 |
35.71 |
8 |
23.53 |
3 |
18.75 |
50 - 59 |
5 |
23.81 |
1 |
7.14 |
11 |
32.35 |
4 |
25.00 |
60 - 69 |
6 |
28.57 |
5 |
35.71 |
3 |
8.82 |
3 |
18.75 |
70 - 79 |
0 |
0.00 |
0 |
0.00 |
2 |
5.88 |
3 |
18.75 |
≥ 80 |
0 |
0.00 |
0 |
0.00 |
3 |
8.82 |
0 |
0.00 |
Total |
21 |
100.00 |
14 |
100.00 |
34 |
100.00 |
16 |
100.00 |
Table 2: Gender and age of patients with colonic carcinoma and control subjects.
These results revealed the distribution of patients according to site of tumor was as: Thirty out of 50 patients had a recto-sigmoid tumor, accounting for 60%.; Seven out of 50 patients had a sigmoid tumor, accounting for 14%.; seven patients had a right sided colonic tumor, accounting for 14%, and six patients had a colonic tumor on the left side, accounting for 12%.
It was obvious that the recto-sigmoid location is the most common site for colonic tumors in patients enrolled in the present study, as shown in figure 2.
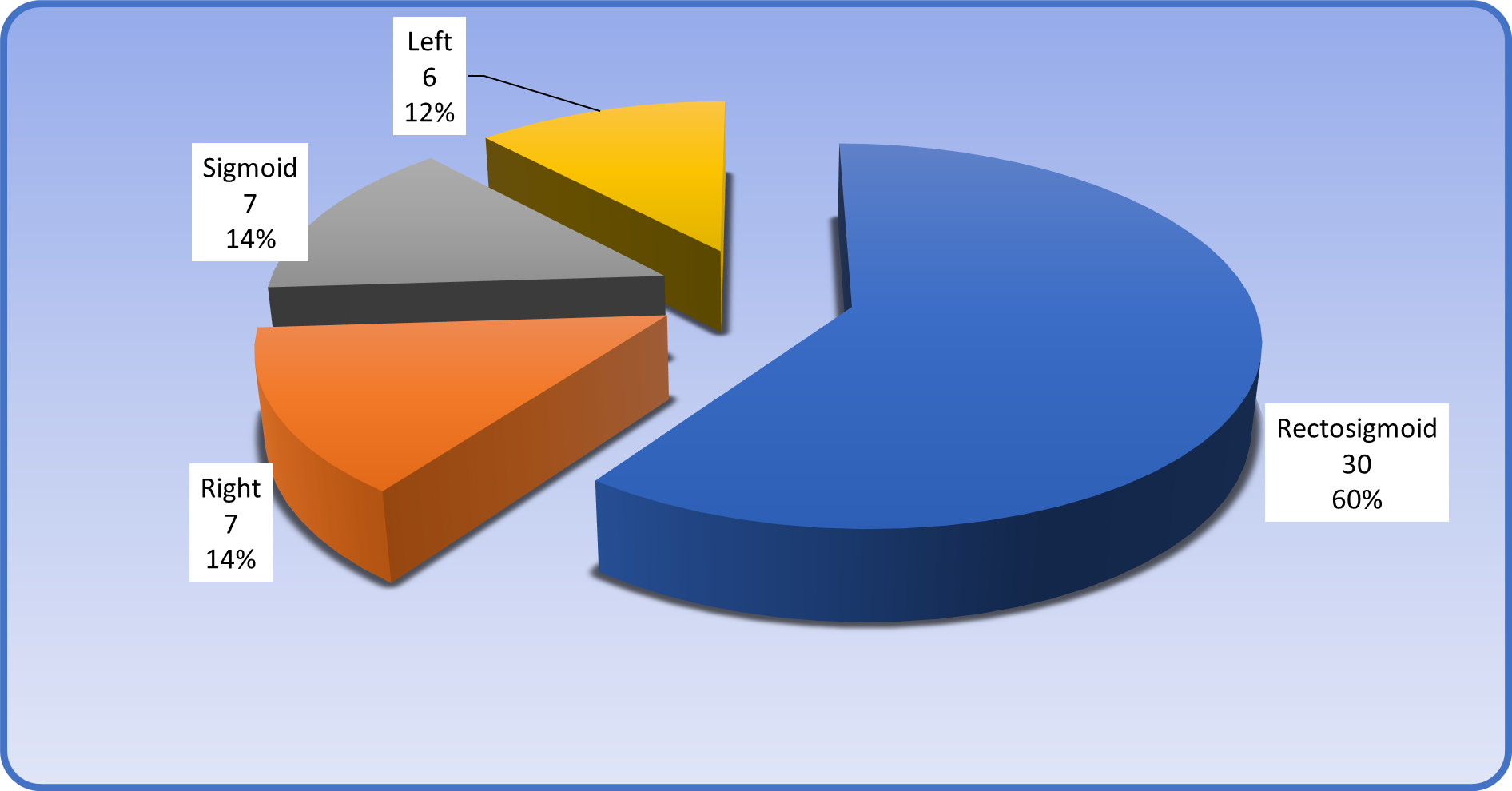
Figure 2: Site of colonic carcinoma.
The mean age of patients according to site was as follows: Patients with recto-sigmoid location had a mean age of 50.6 ± 18.57 years, patients with sigmoid location were 44.57 ± 17.62 years of age, patients with right sided colonic tumor had a mean age of 52 ± 6.45 years and patients with left sided colonic tumor had a mean age of 63.0 ± 8.34 years (Table 3). The results did not show any significant variation, as shown in figure 3.
| Site | N | Mean | SD |
|---|---|---|---|
Recto-sigmoid |
30 |
50.60 |
18.57 |
Right |
7 |
52.00 |
6.45 |
Sigmoid |
7 |
44.57 |
17.62 |
Left |
6 |
63.00 |
8.34 |
Total |
50 |
51.44 |
16.67 |
Table 3: The mean age of patients with colonic carcinoma according to site.
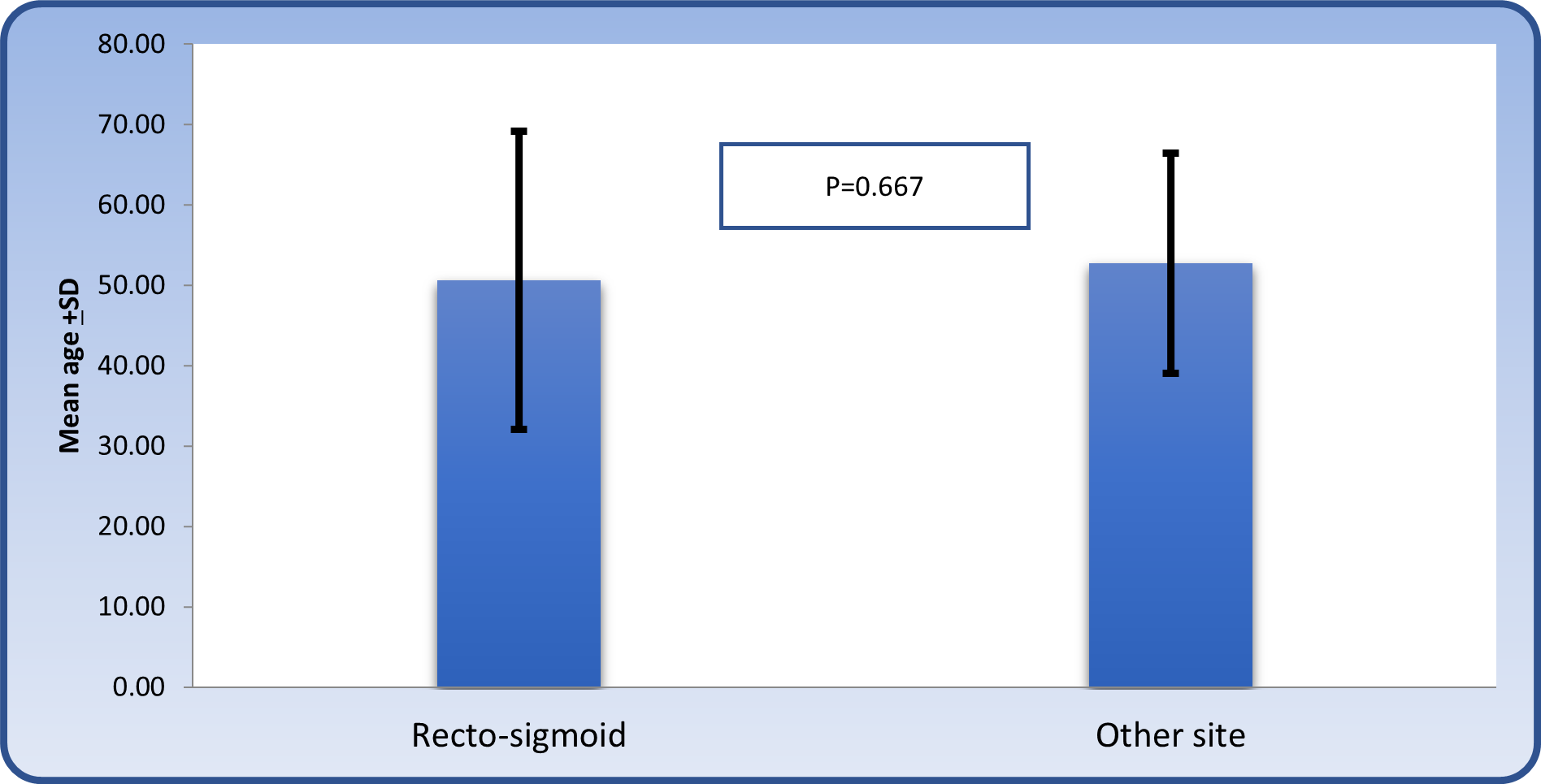
Figure 3: Comparison of mean age between those with recto-sigmoid location and others.
Classification of patients according to the site and genderThe site of colonic tumors in male patients was: 61.76% recto-sigmoid location; 17.65% right sided location; 17.65% sigmoid location and 8.82% left sided location. Additionally, the site of colonic tumors in female patients was: 56% recto-sigmoid location; 18.75 % sigmoid location; 18.75% left sided location and 6.25 % right sided location (Table 4).
| Male | Female | Total | ||||
|---|---|---|---|---|---|---|
| Site | No. | % | No.> | % | No. | % |
Recto-sigmoid |
21 |
61.76 |
9 |
56.25 |
30 |
60.00 |
Right |
6 |
17.65 |
1 |
6.25 |
7 |
14.00 |
Sigmoid |
4 |
11.76 |
3 |
18.75 |
7 |
14.00 |
Left |
3 |
8.82 |
3 |
18.75 |
6 |
12.00 |
Total |
34 |
100.00 |
16 |
100.00 |
50 |
100.00 |
Table 4: The classification of patients with colonic carcinoma according to site of tumor and gender.
Grade and stage of colonic carcinomaThe distribution of patients according to grade of tumor was:
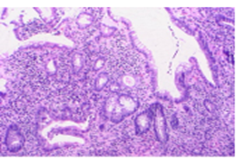
Figure 4: Histological section showing control colonic mucosa (colitis) with well-developed gland, preservation of polarity and presence of simple columnar cells; H & E stain (10X).
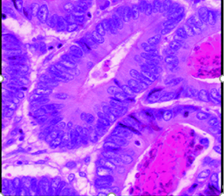
Figure 5: Histological section of colon showing well differentiated grade I adenocarcinoma with well-defined glandular pattern, multilayering, dysplastic changes, loss of polarity, and back to back arrangement; H & E stain (40X).
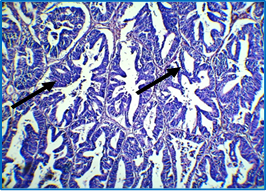
Figure 6: Histological section of colon showing moderately differentiated adenocarcinoma with mixed glandular (right arrow) and solid patterns (left arrow), dysplastic changes, back to back arrangement and loss of polarity; notice absence of goblet cells; H & E stain (40X).
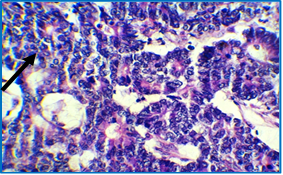
Figure 7: Histological section of colon showing poorly differentiated adenocarcinoma with almost complete solid pattern (arrow), dysplastic changes, back to back arrangement and loss of polarity; H & E stain (40X).
It was clear that the majority of patients exhibited well differentiated grade I tumors (Table 5). According to these findings, it should be mentioned that two of the cases of colonic carcinoma were non-conventional adenocarcinoma: one of them exhibited neuroendocrine differentiation, (Figure 8) and the other one was mucinous (Figure 9).
| Grade | No. | % |
|---|---|---|
I “Well differentiated” |
28 |
56 |
II “Moderately differentiated” |
14 |
28 |
III “Poorly differentiated” |
8 |
16 |
Total |
50 |
100 |
Table 5: The classification of patients with colonic carcinoma according to grade of tumor.
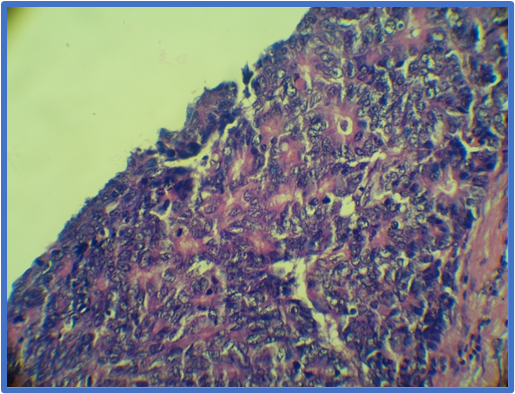
Figure 8: Histological section of colon showing neuroendocrine type adenocarcinoma with organoid pattern of growth and granular cytoplasmic appearance (arrow); H & E stain (10X).
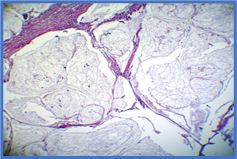
Figure 9: Colonic carcinoma (Mucinous subtype) in which there is extracellular mucin pool (arrow) with floating glandular structures (10X).
The mean age of patients with grade I histological pattern was 56.11 ± 10.40 years, and the mean age of patients with grade II histological pattern was 41.21 ± 19.73 years, whereas the mean age of patients with grade III histological pattern was 53 ± 22.52 years. The Spearman rank test revealed a non-significant correlation between age and grade (Figure 10).
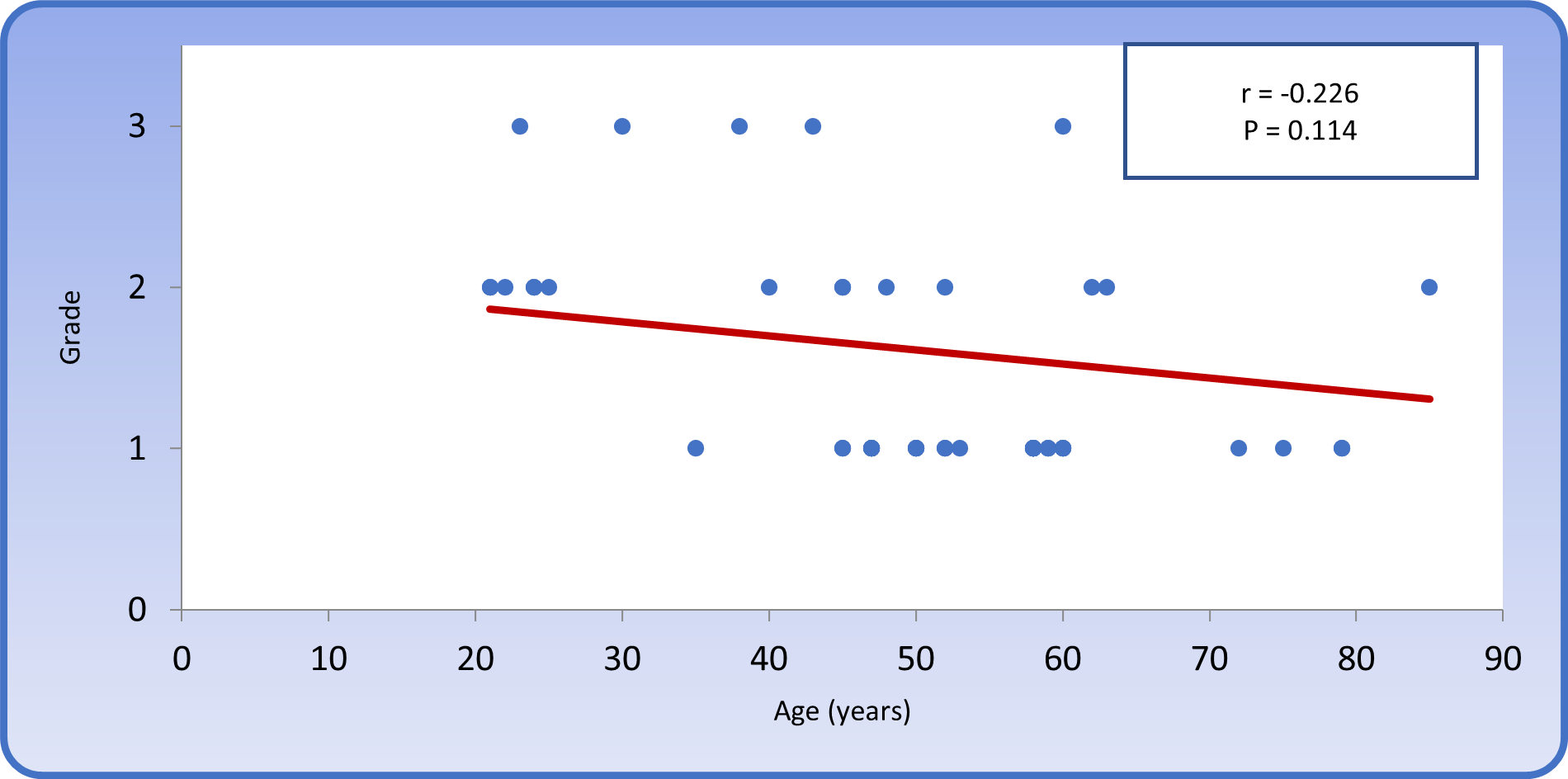
Figure 10: Spearman rank correlation between age and grade in patients with colonic carcinoma.
The distribution of patients according to grade and gender was:
Despite these differences in distribution of patients according to grade and gender, there was no significant association between grade and gender.
The distribution of patients according to grade and site of tumor was:
According to these findings there was no significant association between grade and site of tumor.
The distribution of patients according to stage of colonic carcinoma was:
Most of the patients were in stage II (66%). 50.27 ± 21.3 years was the mean age of patients in stage I disease, 51.7 ± 14.22 years being the mean age of patients in stage II disease, while mean age of patients with stage III disease was 52.17 ± 22.79 years.
Figure 11 showed a non-significant correlation between stage of tumor and age of patients (r = 0.048, P > 0.05).
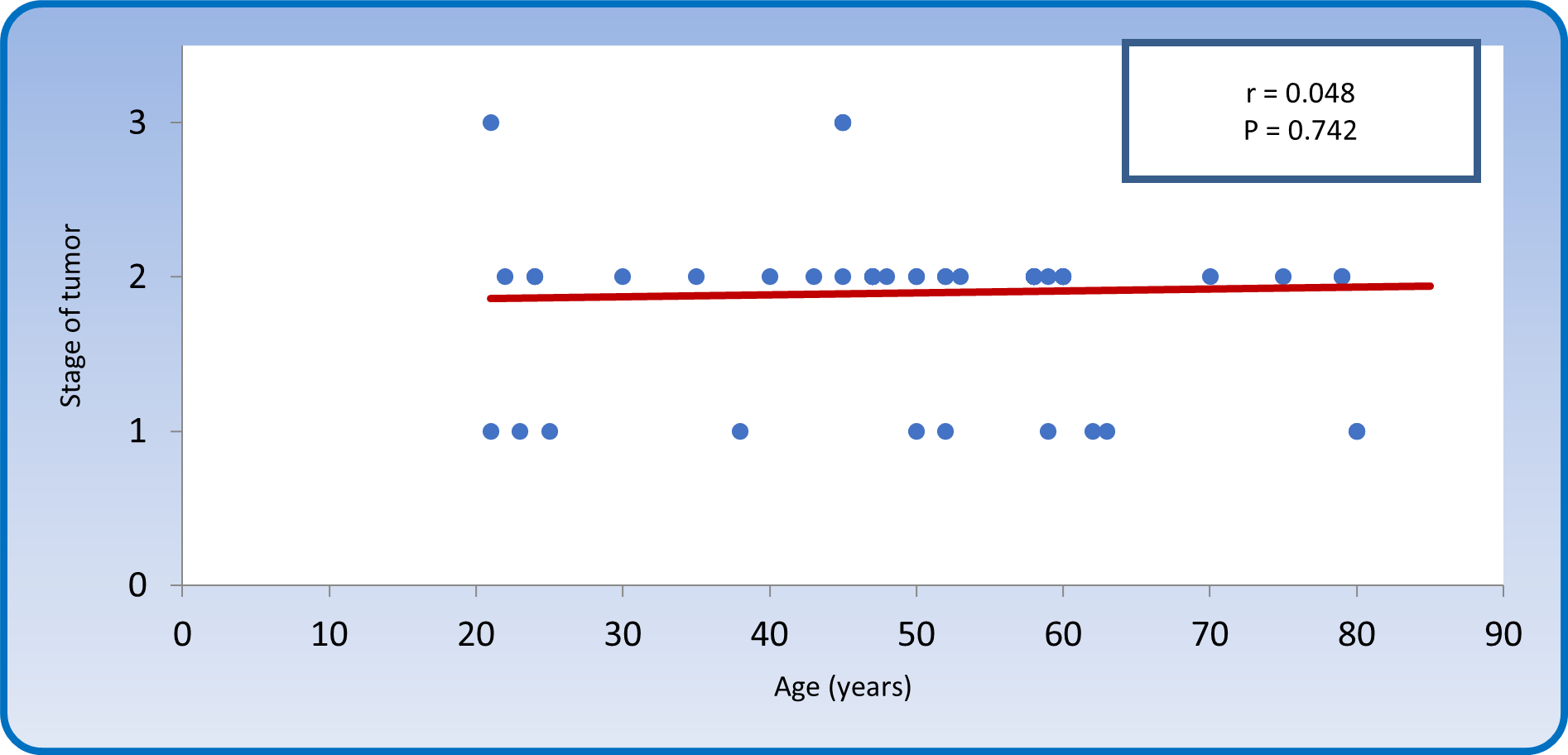
Figure 11: Spearman rank correlation between stage of disease and age of patients with colonic carcinoma.
The distribution of patients according to gender and stage of disease was:
Table 6 shows a significant association between stage of disease and gender of patients (P < 0.05), in such a way that female patients are more liable to advanced stage disease than male patients.
| Male | Female | Total | ||||
|---|---|---|---|---|---|---|
| Stage | No. | % | No. | % | No. | % |
1 |
11 |
32.35 |
0 |
0.00 |
11 |
22.00 |
11 & 111 |
23 |
67.65 |
16 |
100.00 |
39 |
78.00 |
Total |
34 |
100.00 |
16 |
100.00 |
50 |
100.00 |
Table 6: Association between stage of tumor and gender of patients with colonic carcinoma. P = 0.027; Corrected X2= 4.885; DF = 1.
Both grade and stage are ordinal variables; therefore Kendall's tau-b is the best statistical tool to study correlation between them. Kendall's tau-b (Figure 12) yielded a non-significant negative correlation between stage of disease and grade of tumor (r = -0.240, P > 0.05).
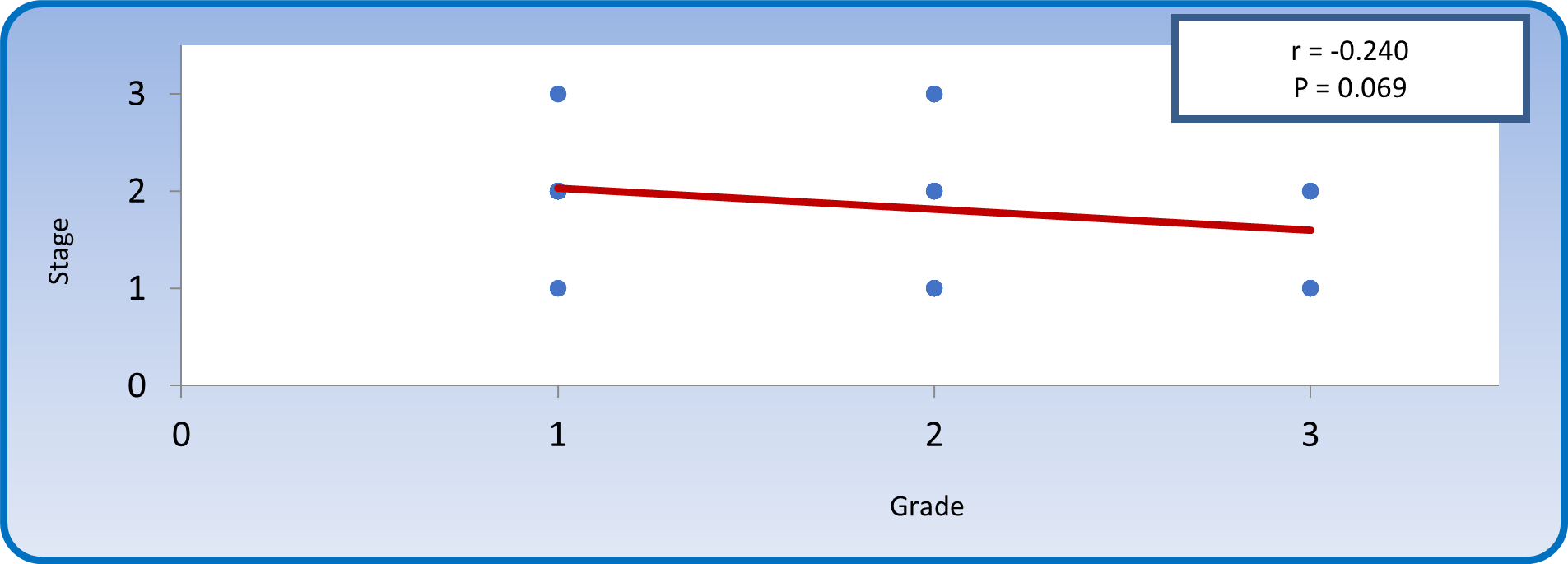
Figure 12: Kendall's tau-b correlation between grade and stage of tumor in patients with colonic carcinoma.
The present study revealed that the mean age of patients with colorectal carcinoma was 51.44 ± 16.67 years and the median was 52 years while the age range was from 21 years through to 85 years. On the other hand, the majority of patients with colorectal carcinoma were more than 40 years of age, accounting for 80%, while 20% of cases were less than 40 years of age. A question comes into mind is that why does colorectal carcinoma affect old age subjects more frequently than young age people?
This question can be discussed based upon the fact that carcinogenesis is a multistep process that involves a sequence of mutational events on the level of oncogenes and tumor suppressor genes. The etiology of cancer in general is attributed to two main general causes, the first one being hereditary germ line mutations in genes controlling cell cycle and growth and the second one is environmental factors.
Environmental factors, specifically the interactions between microorganisms in the colonic mucosa and food digestion products may render the colonic mucosa to become malignant due to mutations in genes. The mean age of patients with colorectal carcinoma was 53.98 ± 14.71 years and the median age was 55 years [25]. These findings are similar to the results of the present study and the minor differences are clearly due to the difference in sample size which was 701 in Iraqi Cancer Registry, while it was only 50 cases in the present study. It was published in the Iraqi Cancer Registry [25] that about 82% of colorectal carcinoma patients are above 40 years of age and that around 18% of patients are below the age of 40 years. Similar findings were found in the present study.
Moreover, another study conducted in Iraq stated that the mean age of patients with colorectal carcinoma was 54.5 years, a finding that solidified the result of the present study [26].
In another study also done in Iraq, the mean age of patients with colorectal carcinoma was 52.4 ± 16.3 years with an age range of 21 - 81 years [27] these findings are comparable to those of the present results.
In a recent study done on 968 cases with colorectal carcinoma in Turkey, the mean age was 58.9 ± 12.6 years which is higher than that of the present study, while the age range was 18-85 years which is comparable to the age range of the present study [28]. This younger age of Iraqi patients with carcinoma in comparison to Turkish patients may suggest the presence of an environmental carcinogenic agent in Iraq that made the progression of colorectal carcinoma faster and to appear in younger age persons.
The discussion section concerning age, which was stated above, came up with an important question that needs to be answered: why do Iraqi people develop CRC younger than nearby countries? This question can be viewed from two points of view; the first one is racial variation in age incidence and the second one is the presence of environmental factors in Iraqi environment that are not present in other countries, exposure to radiation for instance.
Military radiation exposure was found to be a predisposing factor to colorectal carcinoma [29]. An increase in the incidence of birth defects was also observed, in addition to cancers, which were attributed to the use of depleted uranium in Iraq by the US army [30]. Radiation exposure during the first and second Gulf war may explain in part the relatively younger age of colorectal carcinoma patients in Iraq in comparison with nearby countries.
Male to female ratio of enrolled patientsIn the present study, the male to female ratio was 2.12:1. One of the hypotheses of why are males more affected than females is because females have a higher estrogen hormone level, much higher than males. Estrogen was found to be associated with the protection against CRC. Estrogens were found to be important in protecting against the initiation and progression of colorectal carcinoma, and this protective effect is most likely facilitated by estrogen receptor β (ERβ) [31]. The report of Iraqi Cancer Registry [32] stated that the male to female ratio of patients with colorectal carcinoma was 1.23:1, whereas the present study gave a higher incidence in male patients than female patients [33].
Majid., et al. [33] reported that the male to female ratio was 1.6:1, which is slightly higher than that of the present study, but again it emphasizes that males are more frequently affected than females. Al-Humadi [34] reported a male to female ratio of 1.4:1, which is again in accordance with the present study, clarifying a higher frequency of colorectal carcinoma among male patients.
Site of colorectal carcinoma within the colonThe recto-sigmoid region location presented 60% of tumor masses in the current study and 14% of cases were found in the sigmoid region. In combination, rectum and sigmoid area were responsible for 74% of cases. This result should be viewed from two points of view. The first is that screening programs for colonic tumors should make use of colonoscopy procedure as a gold standard test. The second point of view is the question: why do colonic tumors predominantly involve the rectum and sigmoid region? The suggested pathogenesis of colorectal carcinoma is where the answer could be found. It is now well known that most of these malignant tumors arise from premalignant precursors called adenomas [35]. These colon adenomas are categorized into three main types: tubular, villous and tubule-villous adenomas [36]. Multistep genetic and epigenetic mutations occur leading to the progression of villous adenomas to adenocarcinoma [37].
Majid., et al. [33] observed that the rectum was responsible for 35% of the locations of colorectal tumors, similar to the results of the current study. The majority of colorectal tumors involved the rectal region and accounted for about 35% [38] such results were obtained in the current study.
Grade and stage of colorectal carcinomaThe majority of colorectal carcinoma (56%) had a well differentiated grade I histological pattern in this study. It is known that malignant tumors of the large intestines are well-to-moderately differentiated adenocarcinomas, secreting different amounts of mucin [39]. Others studies showed that the majority of colorectal carcinoma cases (64%) were well differentiated grade I lesions [40]. The current study showed similar results.
The present study showed that most of the patients enrolled in the current study (66%) had stage II disease. This can be attributed to the fact that early stage (carcinoma in situ and stage I disease) tumors are often not diagnosed due to lack of proper screening programs like colonoscopy and imaging techniques which can be applied on high risk groups. Most of the patients in the present study had stage II disease is in agreement with many authors [41-43]. The majority of the poorly differentiated cases presented at earlier stages of the disease for tumor differentiation, which may be due to non-expectation of the disease.
From the achieved results of this study, the following can be concluded: There was a significant association between stage of disease and gender of patients and female patients are more liable to advanced stage disease than male patients. Iraqi patients with CRC were at younger ages and had more advanced stages of the disease, presenting mostly in a poorly differentiated type and extra advanced stage than in older patients. These findings make it necessary to conduct a comprehensive awareness program for the control of this type of tumor.
Citation: Homady MH.,et al. “Age and Gender in Relation to Colorectal Cancer in Najef Province: A Histopathological Study". Acta Scientific Paediatrics 5.3 (2021): 72-81.
Copyright: © 2021 Homady MH.,et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.