Roohollah Edalatkhah1, Mohammad Naghibi2*, Farzad Ferdosian3, Zahra Nafei4 and Elahe Akbarian5
1Pediatric Gastroentrologist, Assistant Professor, Department of Pediatrics, Children Growth Disorder Research Center, School of Medicine, Shahid Sadoughi General Hospital, Yazd, Iran
2General Pediatrician MD, Children Growth Disorder Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
3Pediatric Infectious MD, Associate Professor, Children Growth Disorder Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
4General Pediatrician, Associate Professor, Children Growth Disorder Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
5Clinical Psychologist, MSC, Children Growth Disorder Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
*Corresponding Author: Mohammad Naghibi, General Pediatrician MD, Children Growth Disorder Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Received: October 11, 2024; Published: November 21, 2024
Citation: Mohammad Naghibi., et al. “COVID-19 and Growth Indices: Emphasizing Acute Malnutrition in Children”. Acta Scientific Paediatrics 7.11 (2024): 33-40.
Background: Children may become malnourished due to poor diet or nutritional quality, disrupting normal growth. Concerning the COVID-19 pandemic, this study aimed to evaluate the growth indices in suspected or confirmed COVID-19 cases of children in a six-month period during the pandemic.
Methods: In this cross-sectional study, all children below 18 years old with respiratory symptoms and fever were included in the first six months of 2020 in Shahid Sadoughi Hospital, Yazd, Iran. COVID-19 was confirmed by PCR for all patients. Height, weight, head circumference, mid-upper arm circumference, and body mass index (BMI) were all assessed as growth indices. Data were analyzed using SPSS V.20.
Results: A total of 131 children were included, with 51% female. There was no significant difference in gender or nationality between PCR results. Confirmed COVID-19 patients had significantly lower age and mid-upper arm circumference (p-value < 0.05). There was no statistically significant relationship between clinical outcome (death in 10.7%) and growth indices. However, expired patients had longer ICU admission duration (p-value = 0.012).
Conclusions: Acute malnutrition was accompanied by higher COVID-19 infection rates. In order to improve the clinical outcome in pediatric cases, we emphasize improved nutrition, parent education, and additional sensitivity of healthcare workers regarding growth indices.
Keywords: COVID-19; Accounting; Malnutrition
Accounting for about 60% of fatalities of children under five years of age, malnutrition affects about one-third of all children globally, with developing nations reporting the highest prevalence [1-3]. It is in fact the leading cause of immunodeficiency worldwide, affecting a significant proportion of newborns, children, adolescents, and the elderly [4,5]. Malnutrition and infection are closely correlated to infant mortality as inadequate nutrition results in susceptibility to infections. Through modifications in nutrient intake, absorption, needs, and loss of endogenous nutrients, infection can potentially have an impact on nutritional status [6]. Recently, more susceptible children have been malnourished due to poor nutrition and the factors associated with the coronavirus disease 2019 (COVID-19) pandemic and its prevention standards.
Although COVID-19 has infected adults more frequently than children, virtually all age groups have been affected by it [7-9]. COVID-19 has minimal morbidity in children and is generally accompanied by moderate (if any) symptoms, similar to those reported in Middle Eastern respiratory syndrome and severe acute respiratory syndrome. Severe symptoms are usually accompanied by an underlying disease or concomitant medical disorder [8-10]. Herewith, we put an emphasis on malnutrition, as it can hold a bidirectional association with both the COVID-19 epidemic and the syndrome. There have been episodes when efforts to reduce COVID-19 transmission have jeopardized the global food supply chains (GFSCs) and food security. A scarcity of farm labor, restrictions on food accessibility, limitations on the transportation of agricultural commodities, closure of food production facilities, limitations on food trade policies, and delays in the transportation of food products, have all threatened GFSCs, resulting in food insecurity [11]. In the US the percentage of people experiencing severe food insecurity, or food insufficiency, increased from 8% in March to 10% in June 2020 [12]. As of May 2020, the percentage of parents with extremely low food security rose to 29.1% and continued to be high (16.8%) in May 2021 [13]. As a recent study reported, about a third of US families who used infant formula during the first year and a half of the pandemic had difficulty obtaining it, and about the same percentage used undesirable practices, such as diluting formula with extra water or cereal, preparing smaller bottles or keeping the leftover liquid for later use [14]. Due to food scarcity and lockdown procedures, children’s dietary habits have changed, which has resulted in both undernutrition and overnutrition. This change has been especially noticeable in low and middle-income countries, where the epidemic made malnutrition -an already serious public health concern- worse [15]. On the other hand, malnutrition has been shown to strongly affect COVID-19 outcomes [16].
Through this observational study we sought to compare growth indices, as metrics of chronic malnutrition, between pediatric cases with or without COVID-19 infection visited in an emergency department (ED).
Through this cross-sectional study, we included children aged less than18 years, admitted to the pediatric ED at Shahid Sadouqhi Hospital, Yazd, Iran in 2020, presenting with signs and symptoms of respiratory disease, e.g., cough, shortness of breath, fever, gastrointestinal symptoms, headache, restlessness, or Kawasaki-like symptoms. Polymerase chain reaction (PCR) for COVID-19 RNA was performed in addition to a plain radiograph and CT scan of the chest if there were any indication of lower respiratory tract involvement. Based on the result of the PCR test we grouped the patients into suspected COVID-19 (negative PCR) or definitive COVID-19 (positive PCR).
On each participant, we prospectively collected data on demographics (age, gender, nationality), hospital course (hospital length of stay, intensive care unit admission, and clinical outcome), and growth indices including height, weight, head circumference, midupper arm circumference (MUAC). Sex was determined according to the definition by the World Health Organization (WHO) for children under five years old.
Children’s height was measured with a meter with a measuring accuracy of 0.1 cm. Recumbent length was measured in children under two years of age. Standing height was measured in children aged two years or older. We defined moderately stunted, according to the WHO, as a height Z-score between -3 to -2 and severely stunted as a Z-score of less than -3 [17].
Children under two years were also weighed using a beam balance mechanical scale, while older children were weighed using a portable scale. The body mass index (BMI) was also calculated for each patient (weight (Kg) divided by squared height (m2)). According to the BMI, patients were classified as obese (Z-score above +2), overweight (Z-score between +1 and +2), normal (Z-score between -2 and +1), moderate underweight (Z-score between -3 and -2), and severe underweight (Z-score less than -3) [17,18].
Mid Upper Arm Circumference (MUAC) is used to detect acute malnutrition in children. MUAC is commonly measured for children between 12 and 59 months; however, it may even be used for children older than six months who are taller than 65 cm. It is measured at the midpoint between the acromion and the olecranon using a colored plastic non-stretchable strip. While the child’s hand lays parallel to his body, the middle of the arm is marked based on the designated spots. The MUAC strip is a single meter intended to measure the circumference of the middle of the arm. A MUAC of between 115 mm and 125 mm is interpreted as acute malnutrition. Severe acute malnutrition is defined as MUAC of less than 115 mm [19].
A flexible non-stretchable meter was used to determine the child’s head circumference (HC). The meter was placed on the widest part behind the head, and one to two fingers above the eyebrows on the forehead. HC values less than -3 SDs of the median in normal population were classified as microcephaly, between -3 and -2 as abnormal, and more than +2SDs as macrocephaly [17].
Data were analyzed using IBM SPSS Statistics (V20.0, IBM Inc.). We used the Kolmogorov–Smirnov test to evaluate the normality of the data. We also used Mann-Whitney, ANOVA and Chi-Square tests to compare groups. We considered p-values smaller than 0.05 as statistically significant.
We included a total of 131 children with respiratory symptoms and fever, aged less than 18 years with a mean age of 68.4 ± 55.8 months, with 67 patients (51.1%) being female. Furthermore, most patients had Iranian nationality and lived in Yazd (Table 1).
Table 1: Demographic and Growth Index of children aged < 18 years hospitalized with suspected and definite children COVID-19.
We classified patients into four age groups: less than one month, one month to two years, two years to five years, and five years to 17 years. We observed significantly different rates of infection between age groups, with 90.9% positive COVID-19 PCRs from 11 cases aged less than one month. Positive COVID-19 PCR was also reported in 18 cases (75%) aged between one month to two years, 11 cases (47.8%) aged between two to five years, and 27 cases (40.9%) aged between five to 17 years. As a result, a significant inverse relationship was observed between age and COVID-19 infection (P-value = 0.007, Figure 1). However, there was no significant relationship between the clinical outcome and age.
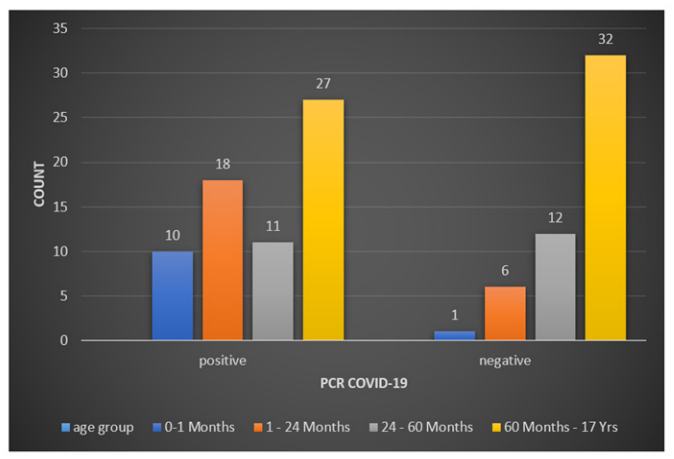
Figure 1
The mean duration for hospital admission was 8.36 days (±10.51), with a median of 5 days. This value for ICU admission also was 3.06 days (±6.58), with a median of 4.5 days. Regarding the clinical outcome, 14 cases (11.7%) expired due to COVID-19 infection. The expired cases aged 52.15 months (±58.09), and the mean duration of ICU admission was 7.2 days (±7.35). There was a significant relationship (P-value = 0.012) between ICU length of stay and mortality.
We identified no significant difference between patients with or without a positive COVID-19 PCR respecting sex, nationality, and hospital or ICU admission duration. In addition, no significant difference was found in mortality rates between the two study groups.
Overall, regarding growth indices, 8.3% were severely stunted, 10.7% were stunted, and 80.9% had normal height/length. Next, 9.1% were severely underweight, 15.2% underweight, and 75.6% normal weight. Classifying according to BMI, however, 8.6% were severely underweight, 23.5% moderately underweight, 46.9% normal BMI, 12.3% overweight, and 8.6% obese. Measuring the HC, 7.3% were microcephalic, 4.8% macrocephalic, and 87.8% had normal HC.
None of the measures proved a statistically significant difference between the two groups, except for MUAP. Six out of six children with severe acute malnutrition were positive for the COVID-19 PCR. Also, three out of three cases with moderate acute malnutrition had positive PCR. Patients with normal values for MUAP (n=71) mostly (52.1%) had a positive PCR test (Table 1). These results indicated a significant relationship between acute malnutrition and COVID-19 infection.
Furthermore, there was no significant relationship between growth indices (MAUC, height/length, weight, BMI, and HC) and the clinical outcome, duration of ICU admission, or gender.
Through this cross-sectional study, we investigated the distribution of growth indices and the prevalence of acute malnutrition in pediatric ED cases with or without COVID-19 in a six-month period in 2020 and its association with the clinical outcome. A positive COVID-19 PCR test was observed in the majority of suspected cases (58%). Significantly higher proportions of younger children had a positive PCR. Among the measured growth indices, MUAP (as a marker of acute malnutrition) was considerably different between patients with or without positive PCR, proving smaller in cases of definite COVID-19. Furthermore, we found no meaningful association between clinical outcome and any growth index or COVID-19 PCR positivity.
Our results did not show a significant relationship between COVID-19 infection and any of the BMI, weight, or height indices (for age). However, MUAC was the only growth index that showed a significantly lower value when there was a definite diagnosis of COVID-19. WHO has recommended MUAC as a means to determine severe acute malnutrition in infants and children [20]. We hence see that acute malnutrition can be associated with COVID-19 infection. We also observed that only 10.6% of the definite COVID-19 cases were obese. Other studies have shown higher values. A study in the USA [21] showed that 37.8% of 576 children with confirmed COVID-19 were obese. Furthermore, according to the CDC report, 33.9% of hospitalized cases of children with COVID-19 were also cases of obesity [22]. We should point to the demographic differences between the populations to explain the observed difference.
We also did not find any case of mortality in the obese patients. High rates have been reported in other studies for mortality in this group. Between February 12 and July 31, 2020, 27.3% of expired patients aged below 21 years (due to COVID-19) in the United States (121 individuals) were obese [23]. It should be noted that the slight difference in patients’ age may explain the observed difference, as adults with obesity are shown to be at more risk of mortality. A meta-analysis by Popkin in 2020 [24] stated that obesity increases the risk of mortality by up to 48% in adults. Of course, obesity can be seen in a bigger proportion of adults than children. A study in New York City 2020, included 5,700 patients hospitalized with COVID-19 and reported a high obesity rate of 41.7% [25]. This further emphasizes the demographic factors that may affect mortality rates in different populations.
In a study conducted in 2019 investigating the rate of malnutrition in hospitalized children and adolescents and their growth indices in Canada, France, Italy, and Turkey, it was reported that 9.1%, 11-12%, 13.2%, and 7.4-27.7% had a BMI less than -2 Z-score, respectively. These values for weight and height were reported in Canada to be 10.4% and 14%, in Turkey 36.6% (weight), and in England 8% 11% [26]. Our findings also detected a prevalence of 19.9% for BMI of less than -2 Z-score, 18.4% for weight, and 12.2% for height. This implies that the rate of malnutrition in the region of our study does not far differ from that of other regions before the COVID-19 pandemic.
Before the COVID-19 pandemic, underweight and wasting children had a prevalence of 11% and 5% among Iranian children less than one year old, respectively [27]. In the current study, the confirmed COVID-19 cases were underweight and wasting in 25.8% and 31.9%, respectively. Also, in a cross-sectional study by Edalatkhah in 2020 including hospitalized children older than the 1-month, [28] the prevalence of wasting, underweight, and stunting was 28%, 27%, and 20%, respectively. Higher rates were observed in our study, at 31.9%, 25.8%, and 27.1%, respectively. This implies that either COVID-19 has been associated with more hospitalization in children with lower weight, or has infected a higher proportion of underweight children compared to other groups.
The prevalence of overweight and obese children has been reported in a meta-analysis by Mansouri [29] to be 9% and 8%, respectively, before the pandemic in Iranian children under the age of five. Another meta-analysis [30] found a prevalence of 7% and 12% for obesity and overweight in Iranian children before the COVID-19 pandemic. A cross-sectional study on 731 preschool children (5 to 6 years old) in Kerman (a neighboring city of Yazd) reported rates of 9.2% and 8.1% as the prevalence of overweight and obese children [31]. In our study, overweight and obesity prevalence in children with COVID-19 was 10.6% and 12.8%, respectively. As the rates suggest, obesity or overweight do not present as likely risk factors for COVID-19 infection or hospitalization in children.
Our findings highlight the role of acute malnutrition in COVID-19 infection in children. Although there have been fewer cases of COVID-19 recently as a result of various preventive measures and vaccination [32], one cannot deny the possibility of future pandemics. Hereby, in order to improve the clinical outcome in pediatric cases, we emphasize improved nutrition, parent education, and additional sensitivity of healthcare workers regarding growth indices.
There was a considerably higher mortality rate in our study compared to others. The results showed that 14 cases (10.7%) died due to COVID-19. While of children under the age of 18, just 6 cases (0.08%) died due to COVID-19 in a study of 7,840 children [33]. Only 121 fatalities were documented in a separate U.S. assessment of 391,814 people with COVID-19, either definitely or suspiciously, or multisystem inflammatory syndrome in children (MIS-C) [23,33]. Our study’s difference in people’s deaths can be attributed to various causes, including challenges and delays in obtaining healthcare services owing to a lack of insurance, childcare, and social health concerns.
There was also no difference in nationality between patients with positive and negative PCR, as 69 patients (58%) with Iranian nationality (n = 119) and seven cases (58.3%) of non-Iranian children (n = 12) had positive PCR. Neither was there any difference between local and non-local residents. Our results go along with those of other studies, as nationality or race is not a differentiating factor in COVID-19 infection [21,34].
There also seems to be no association between COVID-19 infection and gender. Studies have reported fluctuating gender ratios with no specific preference [7,21,35-37]. The results of the current study also showed no significant difference between male and female patients.
The results of this study also revealed a significant tendency for younger children to be infected by the COVID-19 virus. Also, the lower incidence of children with COVID-19 compared to adults could be due to a weaker immune response to coronavirus in this age group [23,38,39], as well as a different expression of angiotensin two converter receptor in children’s respiratory tract compared to adults [35,40-45], or more frequent contact with infected individuals [46].
Our results showed a significant relationship between the COVID-19 positivity test, age, and MAUC. No significant relationship exists between mortality and growth indices (MAUC, height, weight, BMI, head circumference) or age, but the length of stay in the ICU seems to be associated with mortality.
Copyright: © 2024 Mohammad Naghibi., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.