Diaa-Eldin Taha1, Ali Ibrahim1*, Eslam Shokry1, Khaled Magdy Zeinelabden1, Salah Elmekawy1, Ibrahem Ismail Samaha2, Tarek Abdelbaky1 and Hossam Nabeeh1
1Department of Urology, Faculty of Medicine, Kafrelsheikh University, Egypt
2Department of Urology, Faculty of Medicine, Zagazig University, Egypt
*Corresponding Author: Ali Ibrahim, Lecturer of Urology, Department of Urology, Faculty of Medicine, Kafrelsheikh University, Egypt.
Received: September 16, 2024; Published: September 23, 2024
Citation: Ali Ibrahim., et al. “The Effect of Tadalafil 5 mg on Erection and Persistent Storage Lower Urinary Tract Symptoms after Transurethral Resection of Prostate”. Acta Scientific Paediatrics 7.10 (2024): 03-09.
Objectives: Ato assess the effect of early tadalafil 5 mg once daily on persistent storage lower urinary tract symptoms (LUTS) and erectile function after transurethral resection of the prostate (TURP).
Methods: We conducted double-blind, randomized controlled trial in which all patients underwent TURP. Patients were categorized into group A, who received tadalafil 5 mg once daily after surgery, and group B, who received a placebo. Patients were evaluated using the International Index of Erectile Function (IIEF-5) questionnaire to assess the erectile function, the International Prostate Symptom Score (IPSS).
Results: 195 patients were enrolled. The mean (±SD) IIEF in group A was 11.59 ± 1.9, 19.8 ± 1.12, 20.97 ± 0.72, while in group B, 4.88 ± 0.82, 12.86 ± 1.56, 15.32 ± 1.28 at (1, 3, 6 months) post-TURP, respectively (P < 0.001). The mean (±SD) IPSS in group A was 7.31 ± 1.66, 4.46 ± 0.97, 2.33 ± 0.69, while in group B 9.62 ± 3.34, 5.4 ± 1.98, 2.83 ± 1.27 at (1, 3, 6 months) post-TURP, respectively (P < 0.001). The mean (±SD) pre-operative storage symptom sub-score in group A was 3.05 ± 0.78, 1.63 ± 0.49, 0.92 ± 0.67, while in group B was 3.22 ± 0.72, 2.48 ± 0.5, 1.69 ± 0.47 at (1,3, 6 months) post-TURP, respectively (P < 0.001).
Conclusion: Tadalafil 5 mg daily monotherapy after TURP can early improve erectile function, however; it has a slight improvement on persistent storage LUTS post TURP.
Keywords: Tadalafil 5 mg; Storage LUTS; Erectile Function
Men with LUTS due to Benign Prostatic Hyperplasia (BPH) have a higher incidence of ED [1], and LUTS themselves represent an independent risk factor for ED, triggering a significant negative impact on quality of life (QoL). The underlying pathophysiological links between LUTS secondary to BPH and ED are not yet completely understood, even though several determinants are shared by these two clinical entities [2,3].
TURP is considered the gold standard management of BPH despite the new minimally invasive surgical options [4,5]. However, the impact of TURP on sexual function still remains uncertain, Recent studies have shown a negative impact and others have shown improvement in sexual function in patients suffering from ED [6,7].
TURP can cause ED by a variety of mechanisms primarily through a thermal injury that has spread to the cavernous nerves during surgery. Other potential mechanisms include the psychological effect of surgery and the cessation of sexual activity in the postoperative period [8].
Previous studies have reported that some patients had postTURP persistent irritative symptoms and require postoperative medication to treat continuous urinary symptoms. These symptoms are important factors in the patients’ decreased quality of life after surgery. In selecting patients for TURP, predicting the persistence of irritative bladder symptoms is important, but studies investigating the prognostic factors of bladder irritation are uncommon. According to a recent report, postoperative irritative bladder symptoms may remain if there is a severe irritative bladder symptom before the surgery or if there is frequent nocturia [9].
Phosphodiesterase type 5 inhibitors (PDE5-Is) were approved for the treatment of ED, but they have been also demonstrated as highly effective in blunting LUTS in men with or without ED [1013]. Indeed, PDE5-Is act on the relaxation of the bladder neck, and prostate by increasing nitric oxide in smooth muscle, allowing a direct action on micturition phases and not only penile erection [14,15]. Another study demonstrates that once daily 5 mg tadalafil is a potentially effective and safe treatment choice with excellent tolerability for patients with LUTS [16].
Currently, tadalafil 5 mg once daily has been approved for the treatment of LUTS with or without coexisting ED [3]. Another study reported that Tadalafil 5 mg once daily improved LUTS in men without ED by a magnitude similar to that observed in men with ED [17].
The mainstay of medical treatment after radical prostatectomy (RP) to restore spontaneous erectile function remains phosphodiesterase (PDE5) inhibitors, although data from animal studies suggesting that PDE5 inhibitors can prevent smooth muscle apoptosis and fibrosis have not yet been extrapolated to humans because of a lack of standardized protocols. This suggests that a concept of early penile intervention would be promising for those patients who wish to remain sexually active [18].
Tadalafil once daily was most effective on drug-assisted erectile function in men with erectile dysfunction following radical prostatectomy, and tadalafil once daily provided early recovery of erectile function after prostatectomy and possibly protecting from penile structural changes. Unassisted erectile function was not improved after cessation of active therapy for 9 months [19].
To obviate whether it is of value to start tadalafil early after TURP or not? we carried out this study, intending to assess the efficacy of early daily tadalafil 5 mg on persistent storage symptoms and erectile function after TURP.
After institutional board review, the study was carried out between May 2021 and October 2022 in a tertiary hospital. The patients were diagnosed with BPH and underwent TURP either Monopolar or Bipolar. Informed consent was obtained from all patients, and the institutional ethics committee approved the study. Patients were randomized into two groups; the first group (A) patients who received tadalafil 5 mg once daily and the second group (B) patients who received a placebo.
All the patients were sexually active before TURP. Sexually inactive patients were excluded from analyses regarding sexual function due to established limitations of the IIEF in quantifying the sexual function of sexually inactive men.
A pertinent medical history was obtained. The patients were evaluated using the International Index of Erectile Function (IIEF-5) questionnaire to assess the erectile function, the International Prostate Symptom Score (IPSS), storage symptoms were assessed by IPSS storage symptom subscore-15, uroflowmetry, pelvi-abdominal ultrasonography with PVR estimation and laboratory investigations (urine analysis, urine culture, kidney function, complete blood count, coagulation profile, and prostate-specific antigen [PSA]). The evaluation time was unified before TURP and one month, 3 months, and 6 months after TURP.
Patients were divided into two groups using simple randomization (1:1); group A received early tadalafil 5 mg one week after TURP, while the other group (group B) placebo medication (Control group). The randomization was double-blinded (blinded to the patient and surgeon as well). Study completion and reporting will be carried out following the Consolidated Standards of Reporting Trials (CONSORT) (Figure 1).
The sample size was determined a priori using power analysis (G*Power, version 3.1.9.2). Power analysis was conservatively based on three elements: the storage symptoms resolved after TURP, erectile function post-TURP, and LUTs alleviation post-TURP. Type I statistical error of < 5%; and type II statistical error of < 20%. Given the preceding considerations, a sample size of 105 patients in each group was estimated. This sample size provided 80% statistical power and accounted for a 20% dropout rate.
Primary outcome: to assess the effect of early tadalafil 5 mg once daily on persistent storage lower urinary tract symptoms (LUTS) and erectile function after transurethral resection of the prostate (TURP). The secondary outcome is to assess the compliance and adverse effects related to the use of tadalafil.
Data analysis was performed using the software SPSS (Statistical Package for the Social Sciences) version 26. Categorical variables were described using their absolute frequencies and were compared using the chi-square test and Fisher exact when appropriate. Quantitative variables were described using their means and standard deviations or median and interquartile range according to the type of data. To compare quantitative data between two groups, the independent sample t-test (for normally distributed data) and Mann-Whitney test (for not normally distributed data) were used. To compare one variable over two points of time within one group (paired sample t-test for normally distributed data) and (Wilcoxon signed rank test for not normally distributed data) were used. The level of statistical significance was set at P < 0.05. A highly significant difference was present if p ≤ 0.001.
Around 195 patients were enrolled. Patients were categorized into group A (103), who received tadalafil 5 mg once daily, and group B (92), who received a placebo. There is a statistically nonsignificant difference between the studied groups regarding age, BMI, and medical comorbidities (Table 1).
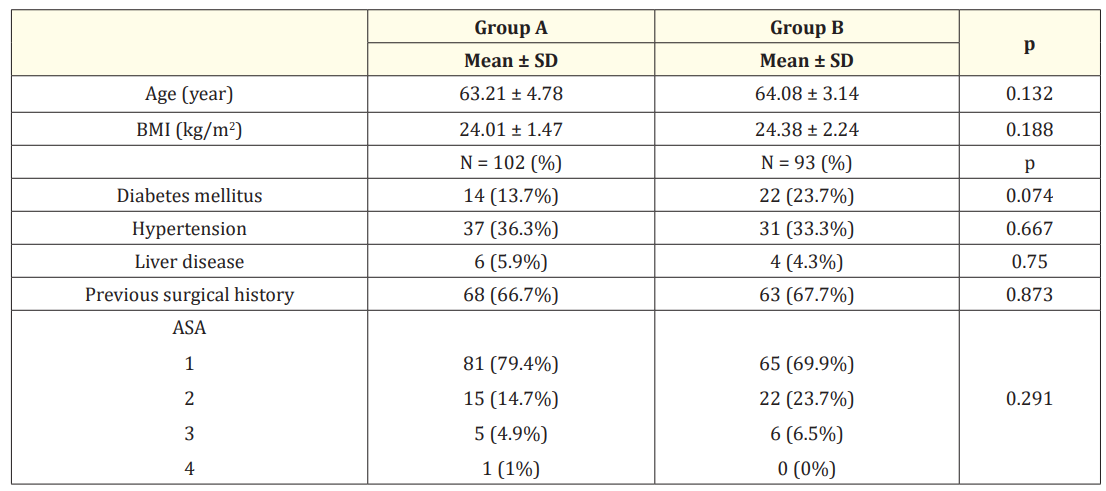
Table 1: Comparison between the studied groups regarding baseline data.
Regarding the erectile function, in group A, there is a significant improvement in IIEF. The mean (±SD) IIEF in group A before TURP was 9.84 ± 2.6 while post-TURP was 11.59 ± 1.9, 19.8 ± 1.12, 20.97 ± 0.72 at (1, 3, 6 months), respectively (P < .001), while in group B, in comparison with IIEF before TURP 10.43 ± 2.81 there is a significant decrease in IIEF at one month after TURP 4.88 ± 0.82, however, a slight improvement in IIEF at (3, 6 months) post-TURP 12.86 ± 1.56, 15.32 ± 1.28, respectively (P < 0.001) (Table 2).
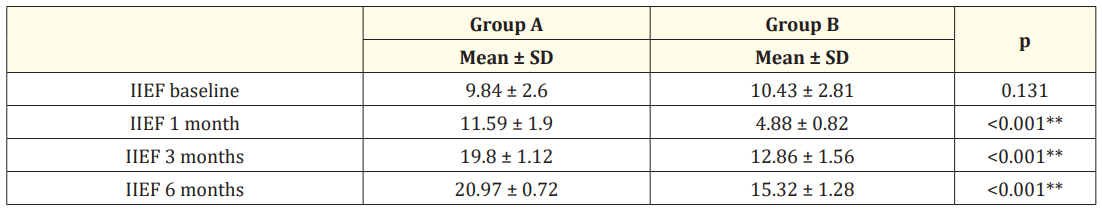
Table 2: Comparison between the studied groups regarding IIEF-5 before TURP and during 6 month follow-up period.
Concerning the overall LUTS, there is a comparable improvement in IPSS, Qmax, and PVR in both groups. The mean (±SD) IPSS in group A before TURP was 24.18 ± 2.41 while post-TURP was 7.31 ± 1.66, 4.46 ± 0.97, 2.33 ± 0.69 at (1, 3, 6 months), respectively (P < .001), while in group B, the mean (±SD) IPSS before TURP was 24.08 ± 2.63 and 9.62 ± 3.34, 5.4 ± 1.98, 2.83 ± 1.27 at (1,3, 6 months) post-TURP, respectively (P < 0.001).
The mean (±SD) Qmax in group A preoperative was 7.54 ± 2.45 while postoperative was 16.05 ± 2.05, 20.32 ± 3.15, 23.41 ± 3.38 at (1, 3, 6 months), respectively (P < .001), while in group B, the mean (±SD) Qmax preoperative was 8.27 ± 2.28 and 15.22 ± 2.0, 19.00 ± 2.1, 21.56 ± 3.59 at (1,3, 6 months) postoperative, respectively (P 06 < 0.001). The (median) PVR in group A preoperative was 100 while postoperative was 40, 20, and 10 at (1, 3, and 6 months), respectively (P 0.2 ), while in group B, the (median) PVR preoperative was 110 and 40, 20, 10 at (1,3, 6 months) postoperative, respectively (P = 0.2).
However, we found in group A that there is a slight improvement in persistent storage symptoms than in group B, The mean (±SD) pre-operative storage symptom sub-score in group A was 8.63 ± 1.82 while post-TURP was 3.05 ± 0.78, 1.63 ± 0.49, 0.92 ± 0.67 at (1, 3, 6 months), respectively (P < .001), while in group B, the mean (±SD) pre-operative storage symptom sub-score was 8.03 ± 0.22 and 3.22 ± 0.72, 2.48 ± 0.5, 1.69 ± 0.47 at (1, 3, 6 months) postTURP, respectively (P < 0.001) (Table 3).
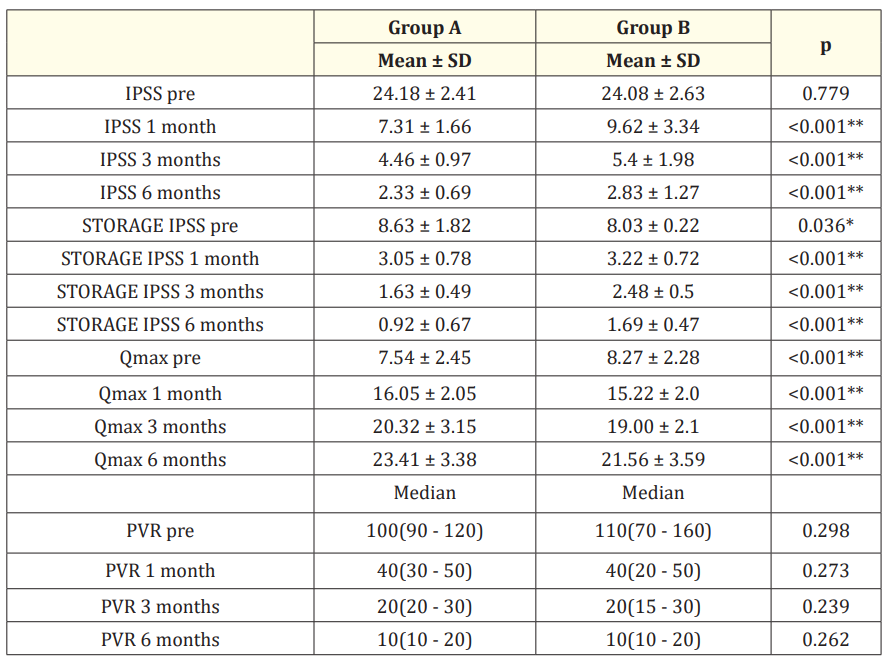
Table 3: Comparison between the studied groups regarding IPSS, Storage IPSS, Qmax, and PVR before TURP and during 6 month follow up period.
Almost all patients were compliant with daily tadalafil. As regard the adverse effect, only 13 patients experienced slight headache and runny nose for a shorter time, Moreover, they didn’t discontinue the tadalafil daily use.
PDE5-I treatment was more effective than placebo treatment in patients with ED after nerve-sparing radical prostatectomy contributing to the recovery of EF after prostatectomy and possibly protecting from penile structural changes [20].
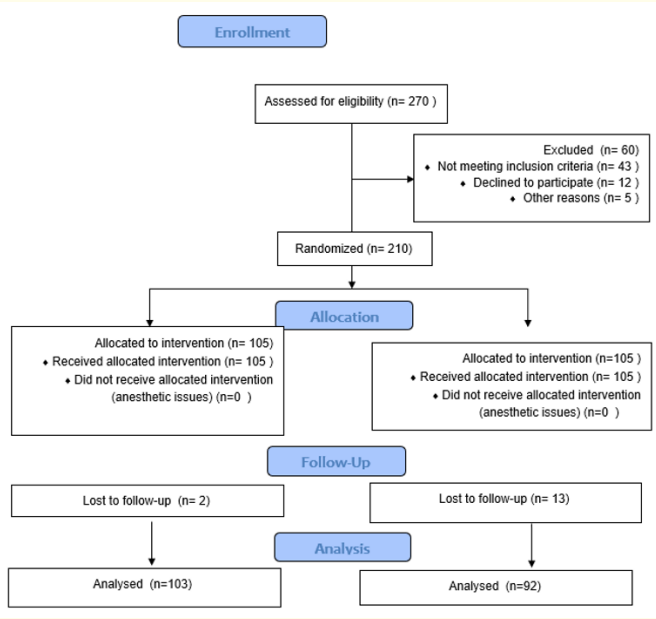
Figure 1: The Consolidated Standards of Reporting Trials (CONSORT).
The same in our study and to the best of our knowledge, this is the first trial comparing the effect of post-TURP early tadalafil-5 mg administration on the persistent storage symptoms and erectile function.
In elderly men, ED and LUTS related to benign prostatic enlargement (BPE) represent a high prevalence comorbid condition with a negative impact on the patient’s QoL and a significant economic burden. It has been established in preclinical and clinical trials that besides aging, several metabolic factors affect the onset and worsening of both ED and LUTS, concurring to penile and nerve alterations and also prostate enlargement and inflammation [21].
TURP is known to be the most effective of all the traditional surgical methods. Once the bladder is free from the obstruction, the thickened bladder wall undergoes atrophy, and its elasticity is restored, thus improving the irritative symptoms. The degree of improvement is reported to be especially high in patients with severe preoperative irritative symptoms [22].
However, a recent study suggested that TURP does not improve irritation symptoms in every patient, and 20% to 30% of patients require medication postoperatively due to persistent irritative symptoms, thus leading to a decrease in quality of life. Significant improvements in LUTS have been yielded by several clinical studies on PDE5-Is. Although the precise mechanism for ameliorating LUTS with PDE5-Is remains unclear, the proposed contributors include relaxing smooth muscle cells in the urogenital tract via the NO/cGMP/PDE5 pathway [23].
In our study, we found that in group A, there is a significant improvement in erectile function, the mean (±SD) IIEF in group A before TURP was 9.84 ± 2.6 while post-TURP was 11.59 ± 1.9, 19.8 ± 1.12, 20.97 ± 0.72 at (1, 3, 6 months), respectively (P < .001), but Regarding overall LUTS, there is a comparable improvement in IPSS, Qmax and PVR in both groups. However, we found in group A that there is a slight improvement in persistent irritative symptoms. The mean (±SD) pre-operative storage symptom sub-score was 8.63 ± 1.82 while post-TURP was 3.05 ± 0.78, 1.63 ± 0.49, 0.92 ± 0.67 at (1, 3, 6 months), respectively (P < .001), as in the first systematic review about the use of PDE5-Is for LUTS, Laydner., et al. reported that PDE5-Is improve IPSS score and IIEF-5 but not Qmax [24].
In agreement with our study, Several RCTs demonstrated that PDE5-Is can significantly decrease IPSS score, ameliorating both storage and voiding LUTS, and improve patients’ QoL [25]. Likewise, in a meta-analysis, IPSS and IIEF scores, but not Qmax, were significantly improved by PDE5-Is [26]. Another study revealed that tadalafil was efficacious and well tolerated in treating ED and LUTS in sexually active men with both conditions. Improvements in both conditions were significant regardless of baseline severity [27].
This study is the first prospective trial of penile rehabilitation with tadalafil 5 mg once a day post-TURP. Our study limitations were the small sample size, so the study should be refashioned in a larger scale of population. Moreover, the study didn’t compare the patients who received tadalafil on demand with the patients who received tadalafil early.
In comparison to placebo, early Tadalafil 5 mg daily monotherapy after TURP can improve erectile dysfunction. We found that tadalafil 5 mg has a slight improvement in persistent storage symptoms post-TURP.
There is no conflict of interest.
Ali Ibrahim: methodology, idea formulation, and reference collection. Diaa-Eldin Taha: review writing and revision, editing the final draft. Eslam shokry: formal analysis and data collection. Ibrahem Ismail Samaha: formulation of hypothesis, Manuscript revision. Salah Elmekawy: processed the translation revision. Hossam Nabeeh: data collection and final revision. Tarek Abdelbaky: supervision.
None.
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Formal consent was signed by the participants for taking part in this research.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Copyright: © 2024 Ali Ibrahim., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.