Bhupesh Dewan*, Sanjaykumar Navale, Siddheshwar Shinde and Janaki Chaudhary
Zuventus Healthcare Limited, Mumbai, Maharashtra, India
*Corresponding Author: Bhupesh Dewan, Zuventus Healthcare Limited, Mumbai, Maharashtra, India.
Received: May 08, 2024; Published: May 26, 2024
Citation: Bhupesh Dewan., et al. “Post-Marketing Surveillance to Establish the Safety and Efficacy of Maxtra®P Syrup in the Symptomatic Treatment of Common Cold in the Indian Pediatric Population”. Acta Scientific Paediatrics 7.6 (2024): 13-20.
Background: The common cold ranks as the most frequently encountered respiratory illness in pediatric medical practice. Although not considered serious, the common cold is widespread and can be debilitating, leading to school absenteeism, disturbed sleep, reduced food intake, and interference with daily routines. It impacts not only a child’s general well-being but also family life as it may require parents to stay home to care for sick children. The main goals of common cold management are the reduction of symptom duration and severity. Effective treatments for common cold include over-the-counter analgesics, nasal saline irrigation, decongestants, and with or without antihistamines.
Aims. To evaluate the safety and symptomatic efficacy of a fixed dose combination of Paracetamol 125mg, Phenylephrine 5mg, and Chlorpheniramine maleate 1mg per 5 ml (Maxtra®P) syrup in Indian children with common cold.
Method. This active post-marketing surveillance study was carried out from February 2021 to November 2022. A total number of 200 children with common cold were prescribed Maxtra®P syrup for patients of age 6 to 18 years for 5 days. Safety assessment was done by analysing adverse events during the trial and assessment of treatment response. Efficacy assessment was based on a reduction in the severity of symptoms and the number of patients achieving complete remission at the end of the study.
Results. Out of 200 children, 93% achieved complete relief from common cold symptoms on day 5. At the end of the study, symptoms such as sneezing, headache, hoarseness, wheezing, difficulty in breathing, and malaise completely disappeared in all children. A statistically significant reduction (p < 0.0001) in symptom score was observed for all the symptoms from baseline to day 5. Maxtra®P syrup was well-tolerated and the results suggest no new safety concerns. Mild adverse events such as drowsiness and dizziness were reported in two children. Patients and investigators rated Maxtra®P syrup treatment as ‘good’ and ‘excellent’ in providing symptomatic relief in 94% and 96.5% of children respectively, suggesting a positive global response to treatment.
Conclusion. Maxtra®P syrup was found to be well tolerated and efficacious in the symptomatic relief of the common cold in the Indian pediatric population.
Keywords: Chlorpheniramine Maleate; Common Cold; Maxtra®P syrup; Paracetamol; Phenylephrine
The common cold is an acute, self-resolving viral illness primarily affecting the upper airways that also may sometimes involve the lower respiratory tract as well [1]. It is the most common acute illness worldwide, especially in pediatric practice [2]. Children typically present with a constellation of symptoms that include sneezing, blocked and runny nose, along with a sore throat, cough, and malaise, with or without a low-grade fever [3]. School children often endure multiple bouts an average of 7 to 10 colds per year [4]. In children, the median duration of cold symptoms is around eight to ten days in those who seek medical care, though this duration can vary, the majority of cases clear up within three weeks. Respiratory viruses, such as rhinovirus, are the primary cause of acute upper respiratory infections. These viruses are predominantly transmitted through contact with the nasal secretions and saliva of infected individuals [3].
While typically resolving on its own, the common cold is widely occurring and can cause significant discomfort and disruption to daily activities [5]. Common colds in children have a profound effect on their general well-being, most of them experience disturbed sleep, loss of appetite, feelings of illness, and interference with play or other daily activities [6]. The effects of the common cold on children extend beyond their health and can affect family life as well. Social burdens associated with the common cold in children include increased rates of illness, missed school days, and parental absence from work due to caring for the sick child, and if not managed properly, these burdens can escalate into more serious health issues [2]. Nasal congestion’s ability to interfere with sleep can lead to fatigue and irritability. The societal and healthcare impact of nasal congestion is substantial [5].
The high prevalence, socioeconomic impact, and limited preventive measures associated with acute respiratory viral infections like the common cold emphasize the necessity for considerable medical attention [2]. Treatment primarily aims to reduce the duration and intensity of symptoms, thereby alleviating discomfort by addressing the most bothersome effects. There are few options for effectively managing symptoms of the common cold, which include nasal saline irrigation, intranasal ipratropium, over-the-counter pain relievers, decongestants with or without antihistamines, zinc, and vitamin C. Complementary and alternative remedies such as camphor, menthol, and eucalyptus oils before bedtime has been shown to alleviate nasal congestion, decrease the frequency and intensity of night-time coughing, and improve the sleep quality for children and parents alike [3].
The common cold is recognized as a multi-symptom ailment characterized by the concurrent presence of several symptoms, typically enduring for a week or longer throughout the illness [4]. Cochrane meta-analyses evaluating the symptomatic treatment of the common cold revealed that monotherapy failed to improve symptoms in children [7]. Multi-ingredient combination products designed for multi-symptom relief aim to safely and effectively address various symptoms concurrently, offering simplicity and convenience when used as directed [4]. The combination of antihistamines with decongestants may help relieve symptoms [8]. Several studies suggest that a triple combination of antihistamine, decongestant, and analgesic offers relief from multiple symptoms in adults, older children with common cold, and allergic rhinitis [8,9]. Urgent attention is required for conducting high-quality clinical trials to showcase the effectiveness and safety of multiingredient combination products in alleviating various symptoms among children.
Therefore, in this study, we have assessed the safety and efficacy of a fixed-dose combination (FDC) of Paracetamol, Chlorpheniramine maleate and Phenylephrine in Indian children where Paracetamol provides relief from body aches, headache, fever, Chlorpheniramine maleate targets rhinorrhea, sneezing, itching, sore throat, cough, and Phenylephrine takes care of troublesome nasal congestion, stuffiness of common cold.
This multicenter, active post-marketing surveillance (APMS) study was conducted from February 2021 to November 2022 at four geographically diverse locations across India, with one clinical trial site representing each region: North, South, East, and West.
Two hundred children displaying symptoms of the common cold were enrolled in the study. The research was conducted in accordance with the protocol and the stipulations outlined in the New Drugs and Clinical Trial Rules (NDCT Rules) of 2019, as well as the guidelines provided by the Central Drugs Standard Control Organization Good Clinical Practices (CDSCO-GCP), the International Council on Harmonization-Guidelines for Good Clinical Practice (ICH-GCP) E6 (R2), and the Declaration of Helsinki.
The study recruited patients aged between 6 to 18 years who had recently developed symptoms of a common cold persisting for more than 6 hours but less than 72 hours. Additionally, children who provided assent to participate in the study were included.
Patients exhibiting hypersensitivity to any ingredient in the formulation, individuals with hepatocellular insufficiency, hepatic failure, or active liver disease, those who had taken an antihistamine, analgesic, or decongestant within 1 day prior to study enrollment, and patients deemed unsuitable for inclusion in the study by the investigator were excluded from participation.
Maxtra®P syrup is a fixed-dose combination comprising paracetamol 125mg, phenylephrine hydrochloride 5mg, and chlorpheniramine maleate 1mg per 5ml syrup manufactured by Zuventus Healthcare Limited, Mumbai Maharashtra, India. For patients aged 6 to 11 years and 12 to 18 years, 5 ml and 10 ml doses of syrup should be administered every 4 to 6 hours respectively.
Informed consent was obtained from parents or legally acceptable representatives of the patients before enrolling them in the study, and informed assent was obtained from pediatric and adolescent patients. During the process of obtaining informed consent and assent, patients were provided with detailed information about the study procedure, study product information, potential risks, and benefits. All eligible patients meeting the inclusion and exclusion criteria were recruited for the study.
During the initial visit (Day 0), patient demographics, detailed medical history, and vital signs such as respiratory rate, pulse rate, and temperature were recorded for all participants. Maxtra®P syrup was dispensed to all patients during this visit, with instructions to take it every 4-6 hours for 5 days.
During the second visit (Day 5), vital signs were examined, adverse events were assessed, and a global assessment of treatment by both patients and investigators was conducted. The severity of symptoms experienced by the patient was assessed on both Day 0 and Day 5 of the study using a subjective rating scale for eleven respiratory tract symptoms.
Concomitant medications that could potentially interfere with study evaluations were prohibited throughout the 5-day study duration.
The primary endpoints included the number of adverse events reported and assessments of the response to Maxtra®P syrup treatment by both investigators and patients at the end of the study. Secondary endpoints comprised a reduction in symptom severity and the number of patients achieving complete remission.
The principal investigator at each study site oversaw the monitoring of adverse effects by inquiring patients or their legally acceptable guardians whether patients have experienced any adverse effects at any point during the study. The reported adverse events were diligently monitored and documented in the Case Report Forms (CRF). Each adverse event was graded as per their severity, and its relationship to the investigational drug was evaluated according to the World Health Organization-Uppsala Monitoring Center (WHO-UMC) scale, categorizing it as certain, probable/ likely, possible, unlikely, conditional/unclassified, or unassessable/ unclassifiable.
Global assessment of treatment response which refers to an overall evaluation or judgment of the patient’s response to Maxtra®P syrup treatment, was also documented. This assessment typically takes into account various factors such as symptom improvement, overall well-being, and any adverse effects experienced. Both investigators and patients/parents were requested to assess the response to therapy on a scale of 0 to 3, with 0 indicating Poor, 1 indicating Satisfactory, 2 indicating Good, and 3 indicating Excellent response. This assessment offers a comprehensive perspective on the treatment’s efficacy from both the patient’s and healthcare provider’s viewpoints.
The severity of common cold symptoms in patients was evaluated using a subjective rating scale encompassing eleven respiratory tract symptoms: runny nose, nasal congestion, sneezing, sore throat, hoarseness, cough, wheezing, difficulty in breathing, headache, fever, and malaise. The Respiratory Tract Symptoms Severity Score was defined on a 5-point scale as follows: 0 for Absent/No Symptoms, 1 for Mild, 2 for Moderate, 3 for Severe, and 4 for Very severe. Complete remission was defined as patients exhibiting zero symptoms of the common cold by the conclusion of the study (Day 5).
Safety was assessed by evaluating the total number of patients reporting side effects. The occurrence of any adverse event reported by the patient was measured as the percentage of patients experiencing side effects.
Data were expressed as mean ± SD or percentage. A comparative evaluation was conducted on the data obtained from each visit. The severity of individual symptoms was evaluated at the final visit by Student’s t-test. Statistical significance was set at p < 0.05.
All 200 enrolled patients were aged between 6 and 18 years. The demographic characteristics of all participants are outlined in table 1.
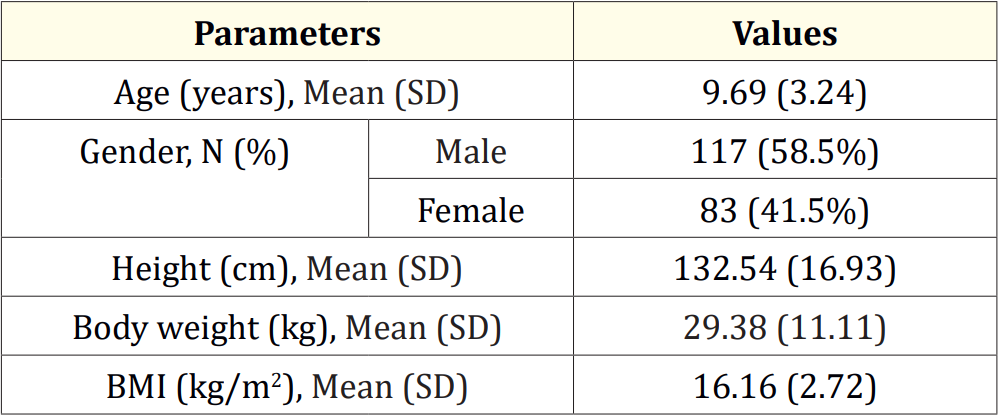
Table 1: Demographics.
Out of 200 children analysed, only two patients had experienced adverse events (AEs) of mild severity such as dizziness and drowsiness. The AEs were resolved without any sequelae and also the causality assessment revealed that they were unlikely related to the study drug.
At the end of the study, the global assessment of treatment response was evaluated based on both investigator’s and parent’s response to the treatment. The response to the therapy was graded by patients and investigators as excellent, good, satisfactory, and poor. The majority of the patients and investigators reported that Maxtra®P syrup provided a good or excellent effect in symptomatic relief of the common cold, in 94% (n = 188) and 96.5% (n = 193) of patients respectively (Table 2 and Table 3).
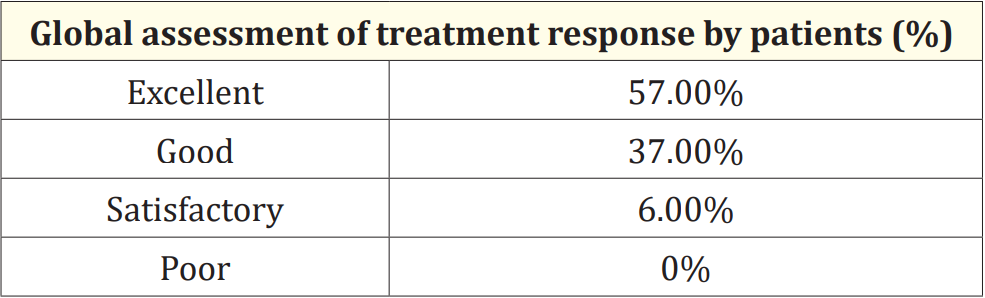
Table 2: Evaluation of Global assessment of treatment response by patients.
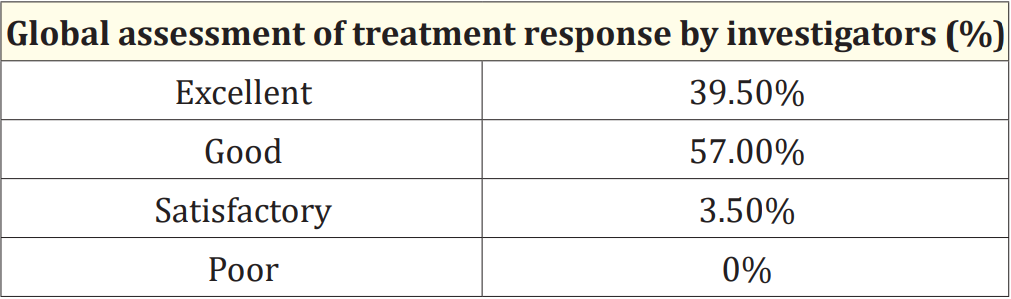
Table 3: Evaluation of Global assessment of treatment response by the investigator.
Overall, the FDC of paracetamol 125 mg, phenylephrine hydrochloride 5 mg, and chlorpheniramine maleate 1 mg syrup was determined to be safe and well tolerated.
All patients presented with at least one common cold symptom at the initiation of treatment. The mean symptom severity scores at visit 1 and visit 2 are depicted in figure 1. A statistically significant reduction (p < 0.0001) in the symptom scores was observed for all common cold symptoms from baseline to Day 5. At visit 2, the mean symptom score was zero for symptoms such as sneezing, sore throat, hoarseness, wheezing, difficulty in breathing, headache, and malaise, indicating complete resolution of these symptoms in all patients after treatment.
Severe or very severe cough was present in 30.5% of enrolled patients at baseline. After treatment with Maxtra®P syrup, 95% of these patients achieved complete recovery from cough. Similarly, 18.5% of patients experienced severe fever at baseline, and all of them experienced relief from fever after receiving the study treatment, except for one patient who still had mild fever on day 5. Patients experiencing severe or very severe symptoms of the common cold, such as runny nose, nasal congestion, sneezing, sore throat, hoarseness, wheezing, difficulty in breathing, headache, and malaise, achieved a 100% reduction in these symptoms after receiving the study treatment.
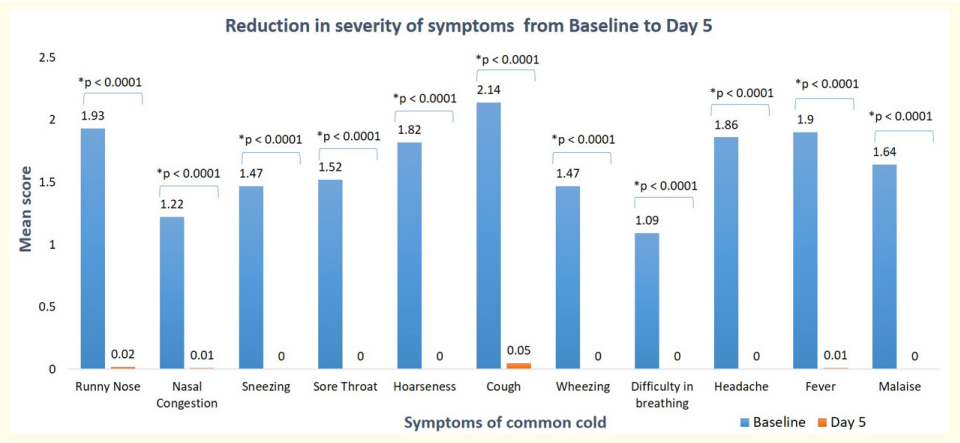
Figure 1: Reduction in severity of symptoms after Maxtra®P treatment.
Out of 200 enrolled children, 186 patients experienced complete recovery from the symptoms of the common cold (Figure 2).
None of the patients had moderate, severe, or very severe symptoms of the common cold at the end of the study treatment.

Figure 2: Patients achieving complete remission with Maxtra®P syrup.
The common cold, although typically mild and self-limiting in nature, remains a global health concern with various negative impacts on individuals’ lives. It is associated with a substantial increase in the number of days spent in bed due to illness, sleep disturbances leading to irritability, reduced activity days, school absenteeism, impaired school performance, reduced appetite, and increased healthcare visits [10,11]. Since there is no cure for the common cold, treatment primarily focuses on relieving the symptoms to improve comfort and facilitate recovery [12].
The combination of multiple active ingredients for common cold relief in a single-dose formula offers comprehensive relief to patients by addressing multiple symptoms simultaneously with a single product, potentially improving compliance with the treatment regimen. Furthermore, it leverages the synergistic effects of different ingredients to enhance overall efficacy. The rationale for consolidating multiple active ingredients into a single formulation for treating the common cold and flu is therefore practical and logical. Indeed, multi-symptom relief combination products such as Maxtra®P syrup containing several active ingredients, offer a safe, efficacious, cost-effective, and convenient means of treating the various symptoms associated with the common cold and flu, provided they are used as recommended [4]. Post-marketing surveillance gives more realistic results as it takes place in real-world settings and provides valuable evidence to both safeguard and enhance the safety of approved drugs [13]. Likewise, this APMS study aimed to evaluate the real-world use of Maxtra®P syrup for treating the common cold in a natural clinical setting among pediatric patients in India.
Our data confirms that Maxtra®P syrup is well tolerated and effective in the symptomatic relief of common cold in the pediatric age group 6 years and above. Out of the 200 children evaluated, complete relief from common cold symptoms was achieved in 93% (n = 186) of patients on day 5. The comparison of mean symptom scores before and after the treatment revealed a significant reduction in intensity for every symptom assessed on day 5. (p < 0.0001). Our findings are in agreement with previous studies conducted in Indian pediatric and young population with the same fixed-dose combination of Paracetamol, Chlorpheniramine Maleate, and Phenylephrine Hydrochloride providing safe and optimum symptomatic relief from the symptoms of the common cold for the treatment of common cold and flu [14-20].
The previous studies on pediatric patients aged 2 years and above, treated with a similar FDC for 5 days, showed a significant reduction in overall symptom scores from baseline to the end of the study (p = 0.015). The FDC was well tolerated and more effective than placebo in the symptomatic treatment of the common cold or flu-like syndrome [14,20]. The total symptom score (TSS) decreased significantly by 41.52% on day 3 and 85.93% on day 5 compared to baseline, without any serious safety concerns [15]. Additionally, published post-marketing surveillance (PMS) data substantiate the safety and efficacy of the FDC of Paracetamol 125mg, Phenylephrine Hydrochloride 2.5mg, and Chlorpheniramine Maleate 1mg for providing symptomatic relief from the common cold and allergic rhinitis in Indian pediatric patients under 1 year of age [18]. A study conducted within the Indian population showed that providing the FDC within the initial 24 to 48 hours of symptom onset resulted in symptomatic relief from influenza [19].
The greatest effect of treatment i.e. 100% reduction in symptom score was observed for sneezing, sore throat, hoarseness, wheezing, difficulty in breathing, headache, and malaise was observed. On day 5 of Maxtra®P syrup treatment, 98%, 98.5%, 99.5%, and 96.5% of patients successfully got rid of their fever, runny nose, nasal congestion, and cough respectively. Maxtra®P syrup treatment was well tolerated and only two mild adverse events were reported such as drowsiness and dizziness. None of the patients reported any major adverse events that necessitated drug discontinuation. These study findings align with previous research, which similarly reported no observed side effects throughout the study period [20]. Our study evaluated children and young patients without associated comorbidities, a factor that substantially reduces the risk of developing adverse effects associated with the combinations. This explains less number of adverse events reported in our study. More than 94% of patients rated global tolerability of Maxtra®P syrup as excellent or good for relief of common cold symptoms while 96.5% of investigators rated global response to treatment as “good or excellent”.
According to Eccles, R., et al. (2014), multi-ingredient combination therapy should be considered a first-line treatment for the common cold, particularly in cases where patients exhibit a range of symptoms. Moreover, they suggest that multi-ingredient combinations do not pose any additional safety risks compared to their respective single-ingredient products [4]. Maxtra®P syrup, which is a multi-symptom relief combination containing three active ingredients, is therefore deemed to provide a safe, effective, cost-effective, and convenient method of treating the multiple symptoms associated with the common cold and flu in Indian children.
Physicians have raised concerns about the safety of prescribing formulations containing paracetamol in combination with decongestants and/or antihistamines for treating the common cold in pediatric patients. To address these concerns and reassure paediatricians, we have demonstrated through our study that paracetamol-containing syrup, specifically Maxtra®P syrup is well tolerated and effective in real-life pediatric clinical practice. Physicians should use these evidence-based observations when prescribing paracetamol-containing syrup for the symptomatic treatment of common cold in pediatric patients.
The outcomes of this study are expected to markedly enrich the current knowledge base, providing valuable insights for healthcare practitioners on the effective strategies for managing the common cold in pediatric patients.
The administration of Maxtra®P syrup provides a near-complete reduction in the intensity of common cold symptoms within few days. Importantly, no new safety concerns were identified during the course of treatment. Maxtra®P syrup offer a safe, effective, and convenient approach in managing multiple symptoms of common cold comprehensively in pediatric age group. This convenience may boost adherence to the treatment regimen, as pediatric patients find it easier to take one medication rather than multiple separate ones. Enhanced adherence supports both patient safety and the optimal effectiveness of the medicines, as it ensures that children are receiving the full therapeutic benefits of the treatment.
Given the good tolerance and efficacy profile of Maxtra®P syrup, it can be regarded as a suitable treatment choice for the symptomatic treatment of common cold in children.
The authors express deep gratitude to the following study investigators: Dr. Ashish R Dhongade (Sant Dnyaneshwar Medical Education Research Centre, Pune, Maharashtra); Dr. Jyotsna Seepana (Government Medical College, Srikakulam, Andhra Pradesh); Dr. Kalpana Dutta (Health Point Multi-Speciality Hospital, Kolkata, West Bengal); Dr. Neeraj Kumar (S.N. Medical College, Department of Pediatrics, Agra, Uttar Pradesh).
None.
The study protocol and related documents were approved by the Institutional Ethics Committee at each study centre. The authors hereby declare that all experiments have been examined and approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the Declaration of Helsinki.
Copyright: © 2024 Bhupesh Dewan., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.