Khondker Qamruzzaman1*, Syed Ahsan Tauhid1, Md Anisur Rahman1, Nadira Dilruba Hoque1, Fauzia Nasreen2 and Md Lutfor Rahman Molla3
1 Associate Professor, Department of Paediatrics, SSMC and Mitford Hospital, Dhaka, Bangladesh
2 Junior Consultant, Paediatrics, Department of Paediatrics, SSMC and Mitford Hospital, Dhaka, Bangladesh
3 Assistant Professor, Department of Paediatric hematology and oncology, SSMC and Mitford Hospital, Dhaka, Bangladesh
*Corresponding Author: Khondker Qamruzzaman, Associate Professor, Department of Paediatrics, SSMC and Mitford Hospital, Dhaka, Bangladesh.
Received: September 11, 2023; Published: September 25, 2023
Citation: Khondker Qamruzzaman., et al. “Clinical Profile and Risk Factors of Enteric Fever in Children: A Study in A Tertiary Care Hospital”. Acta Scientific Paediatrics 6.8 (2023): 30-34.
Background: Enteric fever, caused by Salmonella typhi, is a significant public health challenge in developing nations like Bangladesh, affecting multiple systems and presenting diagnostic complexities due to varied symptoms. Knowledge of clinical characteristics and risk factors is vital for effective case management.
Aim of the Study: The aim of the study was to assess the clinical profile and risk factors of enteric fever in pediatric patients.
Methods: This observational study was conducted at Sir Salimullah Medical College and Mitford Hospital, Dhaka, Bangladesh from January 2021 to July 2022. It involved 134 enteric fever patients from the pediatric outpatient unit, utilizing purposive sampling. Demographic and clinical data were collected via a structured questionnaire, and data analysis employed MS Office tools and SPSS version 23.0 program.
Results: In this study, the majority of participants (49%) were 10-12 years old, with a 2:1 male-to-female ratio. Almost all (98%) presented with fever, followed by vomiting and anorexia in 51% and laboratory results showed 43% with anemia, 32% with lymphocytosis, and 28% with transaminitis. Complications included hepatitis (10%), UTIs (7%), and bronchopneumonia (6%). Risk factors included poor handwashing, ice cube consumption, male gender, and low family income (over 50%). Additional risks included crowding, hand-to-mouth eating, and food sharing (over 30%). Prevalence was higher during the summer and rainy seasons.
Conclusion: Male children are predominantly susceptible to enteric fever, with fever being the most prevalent symptom. The identified potential risk factors for enteric fever include insufficient hand hygiene, ice cube consumption, gender disparities, and economic constraints.
Keywords: Clinical Profile; Risk Factors; Enteric Fever; Pediatric; Salmonella
The global burden of enteric fever is estimated at 21.6 million cases and 200,000 deaths annually, with the highest incidence noted in the South Asian subcontinent [1]. Endemicity in developing countries is attributed to the low standard of living, poor sanitation, poor hygiene practices, contaminated water and lack of universal vaccination. Among children, the common age group affected is between 5 and 19 years, but in some endemic areas of the Asian region, it is also common in children < 2 years [2]. Clinical presentations are non-specific, which may delay diagnosis and treatment leading to fatal complications. Presenting complaints vary from mild constitutional symptoms to severe complications involving multiple organs. Clinical suspicion is pivotal for diagnosis.
Common presentations of enteric fever are fever, vomiting, diarrhea, abdominal pain, cough, headache, and lethargy. The gold standard for diagnosis is blood culture, but 70% of the culture is negative due to injudicious use of antibiotics before admission [3]. Contaminated municipal water supplies and street-vended foods have been implicated in previous risk factor studies of enteric fever [4,5] and are common features of life in South Asian mega-cities such as Dhaka, Bangladesh, a metropolitan area. Enteric fever is the leading cause of bacteremia in children aged <5 years hospitalized in Dhaka, with an annual incidence estimated to be about 18.7/1000 persons [6,7]. Risk factors for enteric fever have been identified in several epidemiologic studies suggesting either water-borne [8,9] or foodborne transmission [10,11]. Whether these factors coincide with those for paratyphoid fever has not been determined. The assumption is that in paratyphoid fever, a higher dose of bacteria is required for infection than in enteric fever; consequently, food is implicated as the major vehicle for transmission of paratyphoid fever, since salmonella bacteria can multiply in food [12]. The objective of this current study was to assess the clinical profile and risk factors of enteric fever in paediatric patients.
This was an observational study that was conducted in the outpatient Department of Pediatrics, Sir Salimullah Medical College and Mitford Hospital, Dhaka, Bangladesh from January 2021 to July 2022. A total of 134 patients admitted to paediatric unit with enteric fever clinically and doing some investigations were enrolled in this study as the study subjects. Properly written consent was taken from all the participants guardians before data collection. Following the study’s inclusion criteria, pediatric patients aged 12 years or younger visited the pediatric OPD with a fever persisting for over 7 days, after excluding other potential sources of infection such as respiratory, nervous system, cardiac, and genitourinary. Those who tested positive in the Widal test (TO titer 1:160 or TH titer 1:160/320) or had a positive blood culture for Salmonella and salmonella Para typhi species were eligible for inclusion. Conversely, the study’s exclusion criteria encompassed patients who left the medical facility against professional advice, as well as cases where consent was not secured. All the demographic and clinical information of the participants was recorded. All data were processed, analyzed and disseminated by using the MS Office tools and SPSS version 23.0 program as needed.
In this study, among the total of 134 participants, 66% were male whereas the rest 34% were female. So, the male- female ratio of the participants was 2:1. Our analysis of participant ages revealed that the 10-12-year-olds formedt he largest group, constituting a significant 49% of the total participants. The age spectrum ranged from 1 to 12 years, with 1-3-year-olds comprising 10% and 4-6-year-olds representing 16% of the participants. Furthermore, 7-9-year-olds accounted for 25% of the study’s participants. When analyzing the distribution of signs and symptoms among all participants, we observed that fever was present in nearly all patients (98%), followed by vomiting and anorexia in 51%. Furthermore, over 25%, 22% and 21% of patients exhibited symptoms such as diarrhea, pallor, and abdominal pain. In more than 17%, 16%, 14% of patients, additional symptoms like cough, hepatomegaly and cholecystitis, and coated tongue were noted. In the realm of laboratory findings, a significant portion of the participants (43%) displayed anemia, while 32% exhibited lymphocytosis, and 28% showed indications of transaminitis. Upon scrutinizing the complications within the entire participant group, the highest occurrence was hepatitis, affecting 10% of the patients, while UTI was observed in 7% and bronchopneumonia was observed in 6% which were also noticeable. Besides, in 2 cases, cholecystitis and in the other 2 cases, encephalopathy was found. In this ongoing study, our observations indicate that over 50% of participants reported behaviors such as not using hand wash, using ice cubes, being male, and having a low family income. Furthermore, in over 30% of patients, factors such as overcrowding, hand-to-mouth eating, and sharing food from the same plate were identified. The prevalence of enteric fever during summer (mid-April to mid-June), and rainy (mid- June to mid-August) seasons was found higher than that in other seasons.

Figure 1: Distribution of participants as per gender (N = 134).

Table 1: Distribution of participants as per age (N = 134).

Table 2: Distribution of participants as per signs and symptoms (N = 134).
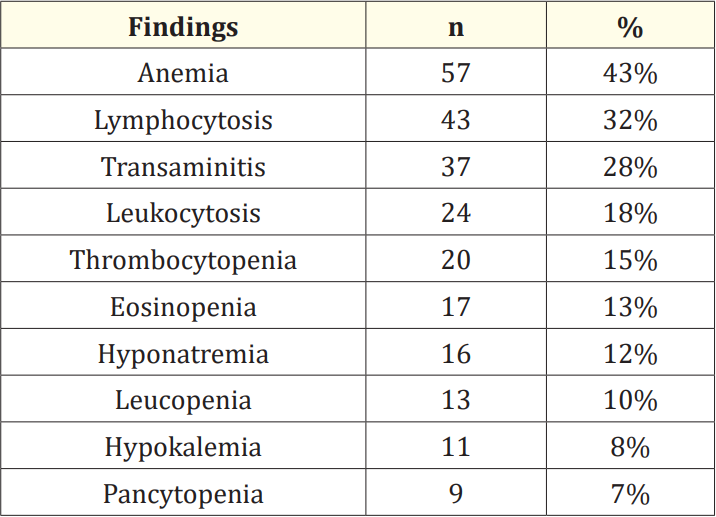
Table 3: Distribution of participants as per laboratory findings (N = 134).

Table 4: Distribution of participants as per complications (N = 134)
UTI: Urinary tract infection..

Table 5: Distribution of participants as per risk factors (N = 134).

Figure 2: Distribution of participants as per gender (N = 134).
The objective of this study was to evaluate the clinical profile and risk factors of enteric fever in pediatric patients. Among the total 134 participants, 66% were male, while the remaining 34% were female. This indicates a higherr epresentation of male participants, resulting in a male-to-female ratio of 2:1. These findings align with those of a previous study [13]. Our analysis of participant ages revealed that the 10-12-year-olds formed the largest group, constituting a significant 49% of the total participants. R Modi., et al. reported the highest incidence of enteric fever in the 6 to 10-yearold age group [14]. On the other hand, another study noted the maximum cases in the age group > 5 years [15]. During the analysis of signs and symptoms among all participants, we found that fever was nearly universal, affecting 98% of patients, followed by vomiting and anorexia in 51% of cases. Additionally, over 20% of patients displayed symptoms like diarrhea, pallor, and abdominal pain. In more than 10% of patients, we also observed symptoms such as cough, hepatomegaly, cholecystitis, and coated tongue. This highlights fever as the predominant symptom seen in almost all cases, while gastrointestinal symptoms like vomiting, diarrhea, and abdominal pain were other significant clinical features, consistent with observations made by other researchers [16,17]. In the realm of laboratory findings, a significant portion of the participants (43%) displayed anemia, while 32% exhibited lymphocytosis, and 28% showed indications of transaminitis. Eosinopenia was detected in 13% of patients, while a higher incidence (72%) was reported in the study by Ramaswamy., et al. [18]. Additionally, the observed rate of transaminitis (28%) was notably lower than that found in the research conducted by Chitkara., et al. (30% vs. 77%) [19]. In our study, upon scrutinizing the complications within the entire participant group, the highest occurrence was hepatitis, affecting 10% of the patients, while UTI was observed in 7% and bronchopneumonia was observed in 6% which were also noticeable. Complications were documented in 21% of cases, with hepatitis being the most common, whereas the Delhi-based study reported only 1.3% complications [19]. In the context of risk factors for enteric fever among all participants in this study, observations indicate thato ver 50% did not use hand wash, used ice cubes, were male, and had low family income. Additionally, in more than 30% of patients, overcrowding, hand-to-mouth eating, and sharing food from the same plate were identified. Notably, the consumption of ice cubes obtained from street vendors could expose individuals to salmonella bacteria, as these bacteria can survive on ice [20]. The presence of low hygienic standards may not only contribute to the transmission of paratyphoid fever but also other foodborne diseases like typhoid or enteric fever [21,22]. In the pediatric population, enteric fever had a higher incidence during the summer (mid-April to mid-June) and rainy (mid-June to mid-August) seasons when compared to other seasons. These findings from our current study can provide valuable insights for future similar studies.
Conducted at a single center with a limited sample size, this study is further constrained by its short duration. Consequently, the scope of the findings may not fully capture the comprehensive landscape of the entire nation.
Numerous challenges persist in effectively controlling and managing typhoid fever in endemic regions. These challenges encompass the need for swift clinical diagnosis and confirmation, as well as growing antibiotic resistance in both S. typhi and S. paratyphi strains. Addressing these concerns necessitates a multifaceted approach, including substantial investments in safe water and sanitation services, community education, regulating antimicrobial prescriptions and over-the-counter sales, and implementing widespread vaccination strategies.
No funding sources.
None declared.
Copyright: © 2023 Khondker Qamruzzaman., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.