Anindita Bose1*, Manisha Banarjee2, Chandan Kumar Shaha3, Shukla Saha4, Arifin Nahar5, Monisha Barman5, Dhananjoy Dey Biplab6 and Arup Kumar Biswas7
1 Specialist, Neonatology, Square Hospital LTD, Dhaka, Bangladesh
2 Professor, Department of Neonatology, Dhaka Medical College Hospital, Dhaka, Bangladesh
3 Assistant Professor, Department of Neonatology, Dhaka Medical College Hospital, Dhaka, Bangladesh
4 Associate Consultant, Department of Paediatric Cardiology, Square Hospital LTD, Dhaka, Bangladesh
5 Senior Specialist, Department of Neonatology, Evercare Hospital, Dhaka, Bangladesh
6 Junior Consultant, Surgery, Bakergonj Upazila Health Complex, Bakergonj, Barisal, Bangladesh
7 Medical Officer, Department of General Surgery, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
*Corresponding Author: Anindita Bose, Specialist, Neonatology, Square Hospital LTD, Dhaka, Bangladesh. Email: drbose.dmc@gmail.com.
Received: July 24, 2023; Published: August 01, 2023
Citation: Anindita Bose., et al. “D-Dimer as A Marker of Early Detection of DIC in Term Newborns with Neonatal Sepsis”. Acta Scientific Paediatrics 6.8 (2023): 01-11.
Background: Disseminated Intravascular Coagulation (DIC) is a common and complex problem from both the clinical and laboratory diagnostic stand-point, with many available laboratory tests being tested and used to be of diagnostic significance. Disseminated intravascular coagulation (DIC) is a life threatening complication of neonatal sepsis. Subclinical DIC may be present in neonatal sepsis. D- dimer is a fibrinogen degradation product, level of which is increased in DIC. So, D- dimer can be used as a diagnostic marker for early detection of in neonatal sepsis.
Objectives: To determine D-dimer as a marker of early detection of DIC in neonatal sepsis in term newborns.
Methods: It was a Descriptive observational study conducted in the Department of Neonatology, Dhaka Medical College Hospital, Dhaka from June, 2018 to November, 2018. Total 50 patients with clinical signs and symptoms of sepsis with positive sepsis screening were enrolled in this study and D- dimer was tested in all of them. Then D-dimer level was compared between culture positive and culture negative sepsis. Also, association between level of D- dimer and platelet count was determined and diagnostic efficacy of D- dimer for early detection of DIC in neonatal sepsis was calculated. AH the enrolled neonates were followed up for next 7 days for subsequent development of clinical DIC. Also the patients with raised level of D- dimer were observed for immediate outcome within 7 days. Data were collected by active participation and interviewing the mother or caregiver. A pre-formed structured questionnaire was used to collect all the necessary data. Data were analyzed in SPSS version 20.0.
Results: Among 17 culture positive patients, 10(58.82%) and 7(41.17%) were from >3 days and <3 day’s age group respectively. Conversely, among 33 culture negative patients 19(57.57%) and 14(42.42%) were from >3 days and <3 day’s age group respectively. Out of 17 patients in culture positive group 11(64.7%) and 6(35.29%) were male and female respectively. Among 17 culture positive patients, 14(82.35%) and 3(17.64%) had D-dimer level >0.5 pig/ml and <0.5 g/ml respectively. On the contrary, among 33 patients in culture negative group, 12(36.36%) and 21(63.63%) proclaimed >0.5 g/ml and <0.5 g/ml D-dimer respectively. The mean D-dimer level in culture positive and negative groups were 1.96(0.1-6.2) and 1.37(0.1-3.2) respectively. Among 17 culture positive cases, 7(41.17%) 5(29.41%) and 4(23.53%) cases had E. coli, Klebsiella and Pseudomonas respectively and whereas only 1(5.89%) case showed Candida. Among 26 patients with raised D-dimer, 17(65.38%) had thrombocytopenia and 9(34.61%) had normal platelet count whereas among 24 patients with normal D-dimer, only 1(4.16%) had thrombocytopenia. For early detection DIC of in neonatal sepsis, sensitivity, specificity, PPV, NPV and diagnostic accuracy of D- dimer were 94.44%, 71.87%, 65.38%, 95.83% and 80% respectively. In culture positive group, 2(11.76%) developed subsequent DIC who had higher D-dimer level. Conversely, none of the culture negative patients developed DIC later irrespective of normal and higher D-dimer level. Out of 14 higher D-dimer patients in culture positive group, 3(21.42%) died and 2(14.28%) experienced persistent organ dysfunction and on the contrary, out of 12 higher D-dimer patients in culture negative group, 1(8.33%) died and experienced persistent organ dysfunction, each.
Conclusion: D-dimer may be used for early detection of DIC without overt clinical features in neonatal sepsis.
Keywords: Disseminated Intravascular Coagulation (DIC); D- dimer; Neonatal Sepsis; PPV; NPV
Sepsis is normally defined as bacteremia in combination with systemic inflammatory response syndrome [1]. Since blood culture has a low sensitivity in neonatal sepsis [2], many studies also included infants with clinical sign of sepsis but a negative blood culture. This condition is normally referred to as clinical, probable or suspected sepsis [3], but has not been sufficiently defined; in addition, the clinical signs that are used vary greatly are poorly evaluated. Sepsis continues to present a significant problem in the neonatal intensive care units (NICU) worldwide [4]. Especially, in this age of advanced neonatal care, sepsis burden assumes even greater significance as extremely preterm infants are increasingly being cared for in the NICUs. Sepsis in premature infants is an important cause of morbidity and mortality [5]. The risk of sepsis increases with decreasing gestational age and birth weight. Neonatal sepsis has traditionally been classified as early-onset neonatal sepsis (EONS), if the onset of infection is in the first 72 hours, and late-onset neonatal sepsis (LONS) if the onset of infection occurs after the first 3 days of life. This classification is helpful in targeting the presumed bacterial pathogens based on the timing of their acquisition. Typically, EONS is attributed to pathogens acquired by vertical transmission from the maternal genital tract and LONS is caused by pathogens acquired by horizontal transmission from the caregivers and environment. Neonatal sepsis is a clinical syndrome characterized by signs and symptoms of infection with or without accompanying bacteremia in the first 28 days of life [6]. Criteria for neonatal sepsis should include documentation of infection in the newborn with a serious systemic illness in which noninfectious explanations for the abnormal pathophysiologic state are excluded or unlikely [7]. Neonatal infections account for the majority of neonatal deaths worldwide. Globally, an estimated 500,000 newborns die each year from serious neonatal infections, which account for about 15% of all neonatal deaths [8]. The incidence of EONS is ~ 0.7-1/1000 live births in developed world [9]. Based on few hospitals based studies performed in developing countries, the incidence of EONS ranges anywhere from 2.2 to 9.8 per 1000 live births [10]. Neonatal sepsis also increases short term and long term morbidities such as necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), patent ductus arteriosus (PDA), prolonged ventilation, neurodevelopmental impairment and prolonged hospital stay. Shatrov., et al. in their mete-analysis showed significant association between chorioarnnionitis and cerebral palsy in preterm and term children [11]. Up to 10% of infants have infections in the first month of life [12]. which are responsible for 30-50% of total neonatal deaths in developing countries [13]. The World Health Organization estimated that there are approximately four million neonatal deaths occur worldwide every year, 98% of which occur in developing countries, particularly in Asia and Africa [14]. A number of organism is associated with neonatal sepsis and bacterial pathogens may vary from one country to another and within a country from one hospital or region to another [13]. Early diagnosis of neonatal sepsis continues to pose a problem to the doctors caring for newborns. It is proposed that coagulation dysfunction one of the many complications of neonatal sepsis is present in around 10% of sick newborns [16]. An intact microcirculation is mandatory for effective oxygen delivery. The widespread formation of micro thrombi in small capillaries in critically Hi patients may occur in any scenario of endothelial injury with subsequent exposure of blood cells and plasma proteins to procoagulant surfaces. In combination with the consecutive consumption of coagulation factors and platelets, this series of events is called disseminated intravascular coagulation (DIC). In some cases, acute hemorrhage also ensues. While activation of coagulation depletes platelets and coagulation factors contributing to clinical bleeding, formation of micro-thrombi causes organ dysfunction. DIC remains an independent pretor of organ failure and death in patients with sepsis. The presence of thrombocytopenia is seen frequently in early sepsis with or without laboratory evidence of overt DIC [17]. Newborns, especially those born preterm, are probably one of the groups with the highest risk among intensive care patients to develop DIC: asphyxia, hypoxia, labile blood pressure and cardiac output, intravascular volume contraction after birth, immature mucosal and skin barriers, as well as multiple invasive procedures and devices, combine to create a dangerous situation. In addition, reserves of pro- and anticoagulant coagulation factors are very limited; thus the overall results are a vulnerable endothelium, early microvascular thrombus formation, and hemorrhage. DIC can be defined as a systemic thrombohemorrhagic disorder seen in association with well-defined clinical situations and laboratory evidence of (1) procoagulant activation, (2) fibrinoiytic activation. (3) inhibitor consumption, and (4) biochemical and clinical evidence of end-organ damage or failure [18]. Subclinical disseminated intravascular coagulation may be present in neonatal sepsis [13]. D-dimer is a fibrinogen degradation product that increases in disseminated intravascular coagulation. D-dimer, formed on cleavage of cross linked fibrin, is a specific marker of fibrinolysis. It is reported that levels of D-dimer increase in patients with sepsis due to fibrinolysis. D-dimer is more specific compared to FDP, which is more sensitive to detect DIC. So, there is a perception that D- dimer can be used as a diagnostic marker for early detection of DIC in case of neonatal sepsis. The main aim of this study is to determine D- dimer as a marker of early detection of DIC in neonatal sepsis.
The general objective of this study was to determine D-dimer as a marker of early detection of DIC in neonatal sepsis in term newborns.
This was Descriptive observational study. The patients were selected purposively. A total of 100 patients were included in this study in two groups. The study was conducted in the Department of Neonatology, Dhaka Medical College Hospital, Dhaka. Bangladesh at June 2018 to November 2018.
Neonates with
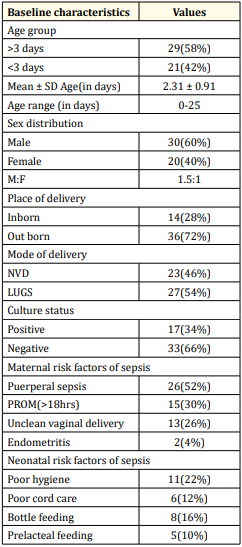
Table 1: Distribution of patients according to baseline characteristic (N = 50).
Table 1 showed that out of 50 patients 29(58%) and 21(42%) belonged to age group >3 days and <3 days respectively. The mean age of the neonates was 2.31 ± 0.91 (age range: 0-25) days. Sex distribution revealed that among 50 patients 30(60%) and 20(40%) were male and female respectively. The male to female ratio was 1.5:1. Out of 50 patients, 14(28%) and 35(72%) were inborn and out born respectively. 23(46%) and 2/ (54%) patients were delivered by NVD and LUGS respectively. Among 50 patients, 17(34%) were culture positive and 33(66%) were culture negative. Out of 50 patients 26(52%), 15(30%), 13(26%) and 2(4%) had maternal risk factors like puerperal sepsis, PROM, unclean vaginal delivery and end metritis respectively. Conversely, 11(22%), 8(16%), 6(12%) and 5(10%) neonates suffered from poor hygiene, bottle feeding, poor cord care and prelacteal feeding respectively.

Figure 1: Column chart showed age group wise patients distribution (N = 50).

Figure 2: Pie chart showed gender wise patients distribution (N = 50).

Table 2: Distribution of patients according to clinical profile of neonatal sepsis (N = 50).
Table 2 showed that among 17 culture positive patients, 15(88.23%), 16(94.12%), 11(64.70%), 7(41.17%) and 4(23.53%) patients presented with reluctant to feed, lethargy, poor reflexes, hypothermia and jaune respectively whereas 3(17.64%) patients presented with hyperthermia, convulsion and respiratory distress each. Only 1(5.88%) patient presented with abdominal distension. On the contrary, out of 33 culture negative patients, 22(66.66%), 17(51.51%), 14(42.42%), 10(30.30%) and 7(21.21%) patients presented with reluctant to feed, lethargy, poor reflexes, hypothermia and jaune respectively whereas 2(6.06%) patients presented with hyperthermia, and respiratory distress each. 4(12.12%) patients presented with abdominal distension. None in the culture negative group had convulsion.
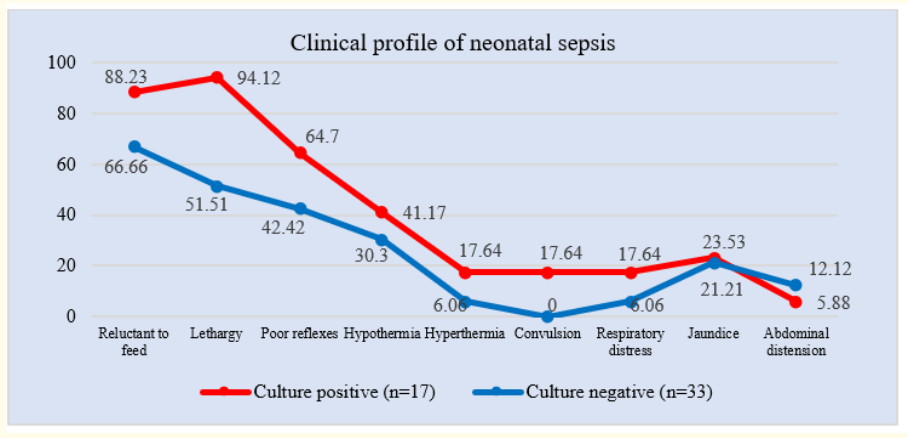
Figure 3: Line chart showed clinical profile of neonatal sepsis (N = 50).

Table 3: Distribution of patients according to sepsis screening parameters (N = 50).
Table 3 showed that among 17 culture positive patients, 14(82.35%), 9(52.94%) and 17(100%) had positive TLC, MicroESR and CRP respectively, whereas, among 33 culture negative patients, 27(81.81%), 15(45.45%) and 31(93.93%) had positive TLC, Micro- ESR and CRP respectively.

Table 4: Distribution of patients of neonatal sepsis according to age (N-50).
Table 4 showed that among 17 culture positive patients, 10(58.82%) and 7(41.17%) were from >3days and <3days age group respectively, whereas, among 33 culture negative patients, 19(57.57%) and 14(42.42%) were from >3 days and <3 day’s age group.
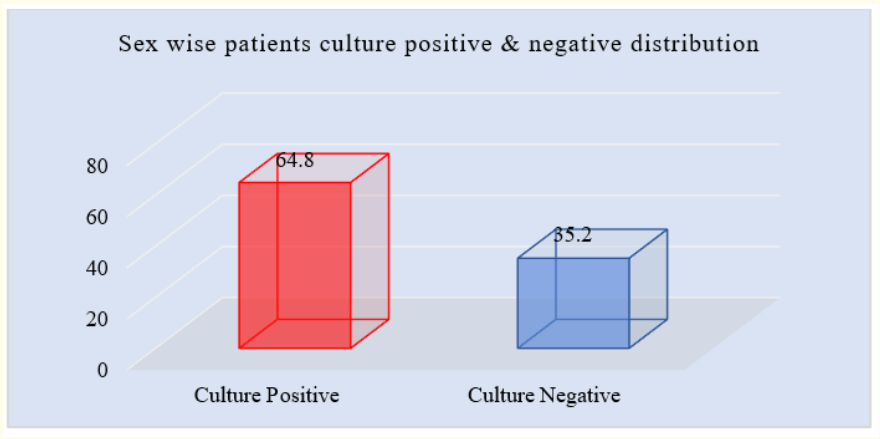
Figure 4: Column chart showed culture positive & negative wise patients sex distribution (N = 50).
Figure 4 showed that out of 17 patients in culture positive group 11(64.8%) and 6(35.20%) were male and female respectively.
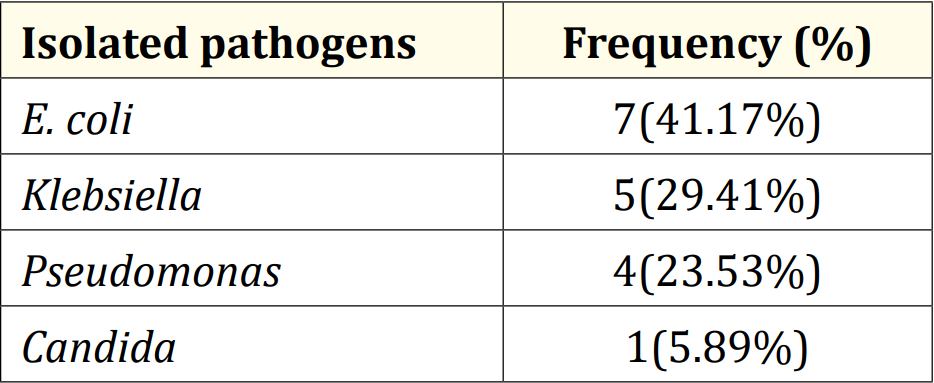
Table 5: Distribution of patients according to isolated pathogens in culture positive group (N = 17).
Table 5 showed that among 17 patients in culture positive cases, 7(41.17%) cases had E. coli, which was subsequently followed by Klebsiella, 5(29.41%). Besides, 4(23.53%) and 1(5.89%) cases had Pseudomonas and Candida respectively.
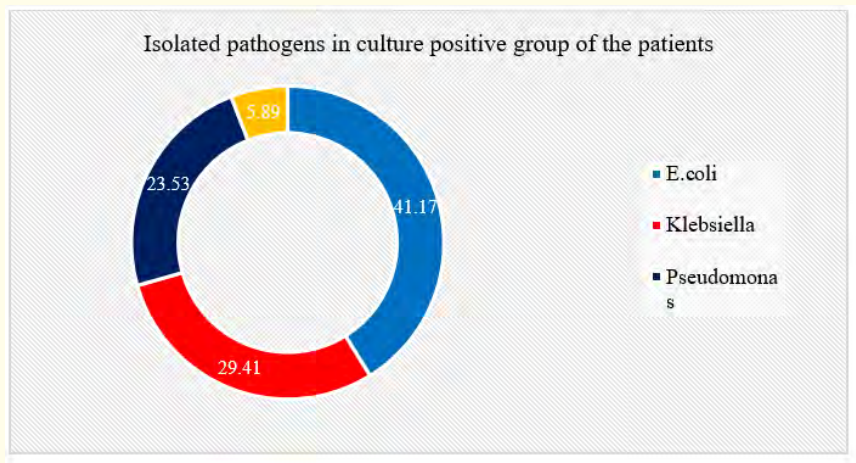
Figure 5: Ring chart showed isolated pathogens in culture positive group wise patients (N = 50).

Table 6: Distribution of patients according to D-dimer status (N = 50).
Table 6 showed that among 17 culture positive patients 14(82.35%) and 3(17.64%) had D-dimer level >0.5 g/ml and <0.5 g/ml respectively. On the contrary, among 33 patients in culture negative group 12(36.36%) and 21(63.63%) had >0.5 g/ml and <0.5 ng/mi D-dimer respectively. The mean D-dimer level in culture positive and negative groups were 1.96 (0.1-6.2) g/ml and 1.37 (0.1-3.2) g/ml respectively.
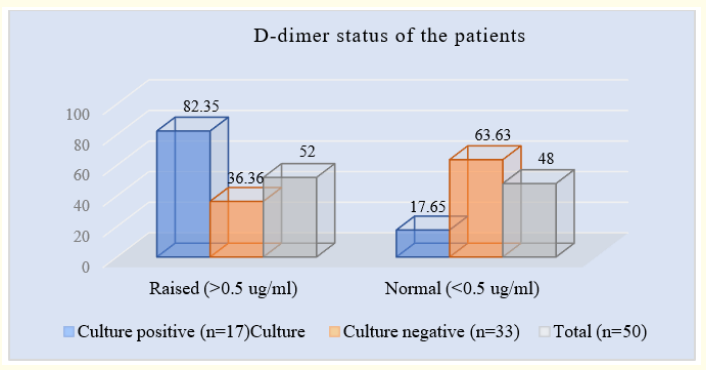
Figure 6: Column chart showed D-dimer status wise patients distribution (N = 50).
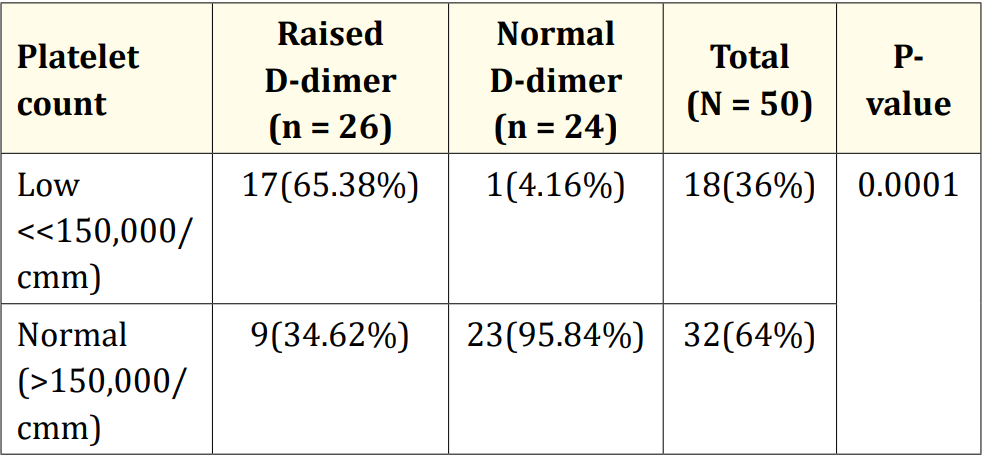
Table 7: Association of platelet count with D-dimer level (N = 50).
Table 7 showed that out of 50 patients of neonatal sepsis, 18(36%) and 32(64%) patients had low and normal platelet count respectively. Among 26 patients with raised D- dimer, 17(65.38%) and 9(34.61%) had low and normal platelet count respectively. On the other hand, among 24 patients with normal D- dimer, 23 (95.83%) had normal platelet count.

Figure 7: Column chart showed platelet count with D-dimer level wise patients (N = 50).
Table 8 shows that the sensitivity, specificity, PPV, NPV and diagnostic accuracy of D- dimer for early detection of DIC in sepsis are 94.44%, 71.87%, 65.38%, 95.83% and 80% respectively.

Table 8: Efficacy of D-dimer in early detection of DIC in neonatal sepsis (N = 50).

Table 9: Association of D- dimer level and subsequent development of clinical DIC (N = 50).
Table 9 shows that out of 17 culture positive patients, 2(11.76%) patients with raised D-dimer level developed clinical DIC.
Conversely, irrespective of normal and raised D-dimer level, none of the culture negative patients developed clinical DIC.
Table 10 shows that out of 14 culture positive patients with raised D- dimer, 3(21.42%) died and 2(14.28%) experienced persistent organ dysfunction. On the contrary, out of 12 culture negative patients with raised D-dimer 1(8.33%) died and 1(8.33%) experienced persistent organ dysfunction. These statistics showed no significant differences (p = 0.809). Rest of the patients with raised D- dimer, irrespective of culture positive or negative sepsis, showed signs of improvement.

Table 10: Distribution of patients according to immediate outcome with raised D-dimer status (N = 50).
Disseminated intravascular coagulation (DIC) is a syndrome wherein the physiologic of clotting and lysing of clot is disrupted. Clinically a patient may demonstrate gross hemorrhage or thrombosis, or both, or may demonstrate only disordered coagulation parameters. Making a firm diagnosis of DIC often has been problematic because the routine laboratory tests address either thrombin generation or plasmin generation, but not both. Detection of D-dimer offers a unique advantage over other laboratory tests for DIC because presence of D-dimer implies both thrombin and plasmin generation. Out of many causes of fatal DIC, neonatal sepsis has always been a cause of concern for the neonatologists throughout the ages. With the causative factors and risk factors being multiple, neonates are always under the risk of being infected and rapidly progressing to many complications associated with sepsis. DIC is one of the life threatening complications of sepsis. Without prompt diagnosis and management of DIC, clinical outcome is often detrimental. In our study, the neonates aged >3 days and <3 days were 29(58%) and 21(42%) respectively. The mean age was 2.31 days (age range: 0-25 days). Besides, the sex distribution showed 30(60%) male and 20(40%) female out of 50 cases. The male to female ratio was 1.5:1. Similar results were observed in a previous study by A Hafsa., et al. [19]. In our study, out of 50 screen positive neonates 17(34%) were found as culture positive and 33(68%) were found as culture negative. Number of culture positive patients was found higher in this study compared to another Bangladeshi study which revealed 13% culture positive neonatal sepsis [20]. This high number of culture positive neonates in our study were detected may be due to meticulous screening of all patients with clinical suspicion of neonatal sepsis and as Dhaka Medical College Hospital is a tertiary care center, lots of out born were usually been referred in this centre. Earlier, culture positive rate of 26% by Ahmed [21] and much higher rates of 51% by Karthikeyan [22] and 64% by Tallur [23] was described. Majority of patients were aged >3 days in both culture positive and culture negative group. It is observed here that 10(58.82%) out of 17 culture positive patients and 19(57.57%) out of 33 culture negative patients came from age group >3 days. On the contrary, 7(41.17%) out of 17 in culture positive group and 14(42.42%) out of 33 in culture negative group came from <3 day’s age group. In a similar study done by Pancham Kumar and his colleagues, similar esuits were observed [24], in another study by A, Hafsa., et al. EONS and LONS were found in 65.38% and 34.82% cases [19] but in our study, LONS (58%) were found more than EONS (42%). Culture positive group showed 11(64.7%) mate and 6(35.29%) female out of 17 participants whereas culture negative group showed 19(57.57%) male and 14(42.42%) female out of 33 neonates in culture negative group. This inclination to male sex was also observed in a previous study [25]. D-dirner values were significantly higher in the culture positive group as compared to the culture negative group (p = 0.002). As in the study by Mautone., et al. [26]. it was found that D-dimer is increased in septic neonates as compared to newborns with mild infection. Arrnengou., et al. [27]. in his study found that normal D - dimer concentration is better at eliminating the diagnosis of than an increased D-dirner concentration at preting sepsis. In this study, D- dimer was found raised in total 26(52%) out of 50 patients with neonatal sepsis. Previously, D-dimer, was found positive in 36(72%) out of 50 patients of sepsis by Sharma, Sikka., et al. [25]. In another study, Kim et al, observed D-dimer positivity in 100% of patients with sepsis [28]. Kinasewifz., et al. also reported D-dimer positivity in 99.7% of patients with severe sepsis [29]. Presence of raised D- dimer in blood is inactive of both thrombin and plasmin generation which in turn inates presence of underlying DIC. In this study, in absence of other causes of DIC, presence of raised D- dimer in patients of neonatal sepsis implies presence of underlying DIC without overt features due to neonatal sepsis. In our study, thrombocytopenia was found in 18(36%) patients of neonatal sepsis, among which 17 patients also had raised D-dimer. Thrombocytopenia was significantly higher among patients with raised D-dimer compared to those with normal D- dimer level. It may be because thrombocytopenia may be present as an early manifestation of DIC [30]. In this study, sensitivity and negative pretive value of D- dimer for early detection of DIC are found higher (94.4% and 95.8% respectively) and specificity and positive pretive values were low. These findings are similar to the findings of Armengou., et al. [27] where the sensitivity and negative pretive value were 97% and 88% respectively and specificity and positive pretive value were 29% and 65% respectively. In our study, DIC had clinically developed in 2(11.76%) patients out of 17 culture positive neonates. A study by Arif, Ahmed., et al. had described that 5.8% of neonates with clinical diagnosis of sepsis had developed clinically overt DIC [17], Hesselvik., et al. studied 53 patients with severe infection; all of them showed positivity for D-dirner but none had overt DIC [31]. Also, in our study, no one in culture negative group irrespective of raised or normal D-dimer level had developed subsequent clinical DIC. The two cases of DIC in our study had much higher D-dimer level and one showed the highest range. But that did not show any significant statistical differences. These 2 patients with raised D-dimer who developed overt DIC also had severe thrombocytopenia. This low number of DIC detected clinically in our study is may be due to ongoing treatment of sepsis by broad spectrum antibiotics and adjuvant therapies for sepsis. Outcome revealed 3(21.42%) patients out of 14 culture positive patients with raised D-dimer died and 2(14.28%) developed persistent organ dysfunction. On the contrary, one (8.33%) died and had persistent organ dysfunction, each, among 12 culture negative with raised D-dimer neonates (P = 0.809). The mortality found by Sharma, Sikka., et al. was 10% while 72% of patients were positive for D-dimer but none of these patients had clinical manifestation of DIC [25]. Mortality was observed in 4(8%) cases in total out of 50 neonates that was a little higher than the study by A. Hafsa [18]. This may be due to, in DMCH most critical patients are referred from the whole country after spending long valuable time following delivery. Among 17 culture positive cases, in this study, 7(41.17%) showed growth of E. coli, which was subsequently followed by Klebsiella 5(29.41%), Pseudomonas 4(23.53%) and Candida 1(5.89%) respectively. No gram positive organism was isolated during this study. Findings by Aftab, Iqbal., et al. revealed that among gram negative isolates E. coli was the commonest organism followed by Klebsiella which is similar to our results [32]. According to Vergnano, Sharland., et al. the pathogens most often implicated in neonatal sepsis in developing countries differ from those seen in developed countries. Overall, Gram negative organisms are more common and are mainly represented by Klebsiella, Escherichia coli, Pseudomonas, and Salmonella. Of the Gram positive organisms, Staphylococcus aureus, coagulase negative staphylococci (CONS), Streptococcus pneumoniae, and Streptococcus pyogenes are most commonly isolated [33]. The predominance of gram negative organism correlated the findings of other workers [34]. Study by AH Movahedian., et al. has described 72.1%-gram negative organism isolation among the proven sepsis, where Pseudomonas was topping the list [35]. another study by A. Hafsa has found Klebsiella as the most common isolated organism. Some authors [19]. have reported increasing trend of CONS in neonatal sepsis. Studies from different countries report CONS as the predominant organisms in LONS. In other studies, gram positive bacteria such as S. aureus and group B streptococcus (GBS) were found to be the most common isolates in neonatal septicemia [36]. But in this study no gram positive organism was found. Proinflammatory responses to S. epidermidis are dependent on gestational age [37]. The variance of bacterio Sogic growth is not clear but it may be the population difference with regard to the maternal colonization rate, nosocomial acquisition of bacteria by the mother and the neonate, genetics of the immune response in selected population groups [10].
In a previous study in Bangladesh it was observed that Acinetobacter (18%) and Pseudomonas (7.95%) spp. were the most common gram negative organisms in early onset neonatal sepsis. In late onset sepsis, Acinetobacter (9.08%) was the predominant organism. In general, Acinetobacter was the 2nd most common isolates (27.27%) causing neonatal sepsis. In our study, no Acinetobacter was isolated during the study period among the study group. This finding was may be due to exclusion of other risk factors of sepsis like perinatal asphyxia, prematurity. The pattern of isolated organisms slightly differs from the findings in Iran [35]. where Pseudomonas Aeruginosa was the most common cause of neonatal sepsis followed by Klebsiella spp. and E. coli. In a similar study from Bangladesh, Nepal and Pakistan, E. coli was the leading cause of neonatal sepsis followed by K Sebsiella spp [32]. Klebsiella organism was isolated from the sample of our neonates. In western countries, though E. coli was showed as the commonest organism but simultaneously group B streptococci (GBS) was also isolated there, which was not observed in our study [37]. In Middle East 43% of neonatal sepsis responsible for Pseudomonas spp, while some other group showed 6% predominance of K Sebsiella spp. [34]. Our findings were different from these findings of several previous Bangladeshi studies.
D-dirner was found significantly raised among newborns with culture positive sepsis. D-dirner has high sensitivity and negative pretive value (94% and 96% respectively) for early detection of DIC in neonatal sepsis.
It was a single center short duration with small sample size descriptive observational study. So the findings of this study may nor represented all over the country.
A multicentered study in the divisional/tertiary hospitals among a large sample over a long time is recommended.
Copyright: © 2023 Anindita Bose., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.