Ngo Yamben Marie-Ange C1,2*, Tsiagadigui Tsiagadigui Jean Gustave1, Nseme Etouckey Eric1, Nana Chunteng Theophile3, UM SAP Suzanne4, Muluem Kennedy1, Batchom Daudet5, Mohamadou Guemse1, Mballa Alexandra4, Manga Alexandre2 and Nguefack Seraphin2,4
1 Faculty of Medicine and Biomedical Sciences of the University of Yaoundé I, Department of Surgery, Cameroon
2 National Centre for the Rehabilitation of Persons with Disabilities in Yaoundé, Cameroon
3 Faculty of Health Sciences, University of Buea, Cameroon
4 Faculty of Medicine and Biomedical Sciences of the University of Yaoundé I, Department of Paediatrics, Cameroon
5 Faculty of Medicine and Pharmaceutical Sciences of the University of Douala, Cameroon
*Corresponding Author: Ngo Yamben Marie-Ange C, Faculty of Medicine and Biomedical Sciences of the University of Yaoundé I, Department of Surgery, Cameroon.
Received: February 13, 2023; Published: March 29, 2023
Citation: Ngo Yamben Marie-Ange C., et al. “Characteristics Of Pediatric Cerebral Palsy in An African Rehabilitation Centre”. Acta Scientific Paediatrics 6.4 (2023): 45-50.
Introduction: Cerebral palsy (PC) is a serious condition, an important cause of psychomotor delay in children with many etiologies. A better knowledge of this polymorphic condition is necessary in order to define prevention strategies and initiate early and adapted management to improve the quality of life of these patients.
Objective: The aim of our study was to describe the epidemiological, clinical and prognostic aspects of cerebral palsy severity in our context.
Patients and Methods: We conducted a cross-sectional, prospective and analytical study at NCRPD for 10 months. All children up to 15 years of age with cerebral palsy were included and followed for at least 3 months. Patient autonomy was assessed according to the Gross Motor Function Classification System (GMFCS). History, epidemiology, clinical and prognostic data were collected and analysed using EPI INFO Version 3.5.1.
Results: We included 92 patients with male predominance (55.4%). Children aged 1 to 5 years were the most affected (46.7%). Neonatal asphyxia (48.91%) was the most common pernatal cause and spastic tetraparesis, the predominant clinical form (71.7%). Osteoarticular complications were found in 22.9% of patients. Oral dyspraxia (72.8%), epilepsy (70.7%) and language disorders (65.2%) were the main comorbidities. Prognostic severity factors identified were ≥ IV GMFCS, bowel movements, language disorders and severe to profound mental retardation.
Conclusion: Cerebral palsy is a polymorphic clinically expressed pathology. The existence of certain comorbidities aggravates the severity and increases the disability of these patients. This preliminary study will serve as a basis for refining the management of these patients.
Keywords: Cerebral Palsy; Tetraparesis; Development Quotient; Walking; Disability; GMFCS.
Cerebral palsy (PC), formerly called «cerebral palsy» is the leading cause of psychomotor retardation in children in Cameroon [1] and worldwide [2]. It represents 18.35% of the reasons for neuropediatric consultations [3]. Of polymorphic clinical expression, it is at the origin of often significant motor disabilities that would be the consequence of non-progressive and permanent brain lesions occurred in the fetus or before the age of 2-3 years [4]. Elle would affect about 1.500 children per year, a third of whom will not walk before the age of 5 [5]. Because of the comorbidities frequently associated [2], multidisciplinary management is necessary in order to develop prevention strategies, to improve the quality of care of these patients and to reduce the severity of the related disability. The aim of our study was to describe the epidemiological, clinical and prognostic aspects of the severity of cerebral palsy in our context.
We conducted a cross-sectional and analytical study at the National Centre for the Rehabilitation of Persons with Disabilities (NCRPD) in Yaoundé over a period of 10 months (FebruaryDecember 2020). The NCRPD was chosen because it is a care center specialized in rehabilitation and endowed with varied expertise (pediatric and adult orthopedists, pediatric neurologist, neurologist, ENT, psychomotor therapists, occupational therapists, physiotherapists, speech therapists, ortho-prosthetists, psychotherapists, carers and social workers).
The main objective of this study was to describe the epidemiological, clinical and prognostic aspects of severity of cerebral palsy in our population.
We included all children of both sexes, aged 0 to 15 years and followed at the NCRPD for at least 03 months whose diagnosis of CP, all clinical forms, had been confirmed by the European paediatrician. All patients lost to follow-up were excluded. The sampling was consecutive and exhaustive.
The variables studied were prevalence, age, sex, obstetric and perinatal history, anthropometric data and WHO Z-score, Denver scale developmental quotient (0-6 years) and Binet-Simon test (> 6 years) and patients’ level of disability by Gross Motor Function Classification System (GMFCS) (Appendix 1). Disability was mild for Level I, moderate for Levels II and III, severe for Levels IV and V. Infants less than 18 months of age were not included.
Data was collected in CSPRO version 7.1 and Microsoft Excel 2010. For statistical analysis, theChi2 test was used with a significant p-value < 0.05.
Over the study period, 505 children were received, 94 of whom had cerebral palsy; with a hospital prevalence of 18.6%. 2 parents refused to participate in the study, so we retained 92 patients among which 51 boys (55.4%) and 41 girls s (44.6%) for a sex ratio of 1.24. The most affected age group was [1-5] years (46.7%) (Figure 1).
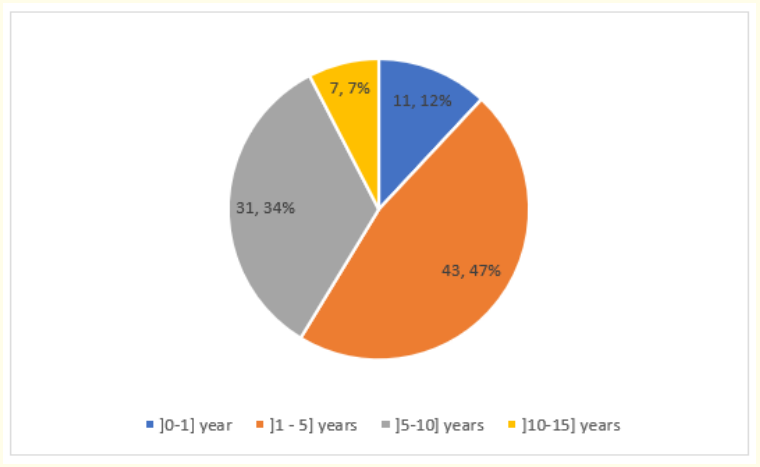
Figure 1: Age distribution of patients.
The median age of onset of symptoms was3 months [1-6 months]. The median time to consultation after the onset of the first symptoms is 2 months [1-12 months] while the median time to diagnosis is1 month [0-4 months].
In the perinatal history (Table 1), it appears that 39.1% of the mothers of children with CP had had a prenatal infection and 82.6% had given birth vaginally. For one in two children, Apgar’s score was depressed (50%) while 46.7% had experienced neonatal seizures. Neonatal asphyxia was implicated in 48.9% of PCs.
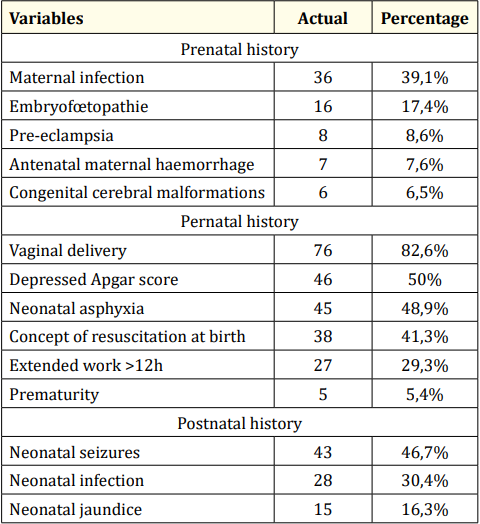
Table 1: Perinatal history found in children with cerebral palsy.
These children were mainly taken for consultation for walking and/or posture disorders (78.3%)), language and/or speech disorders (65.2%) and delayed walking (63%) (Table 2).
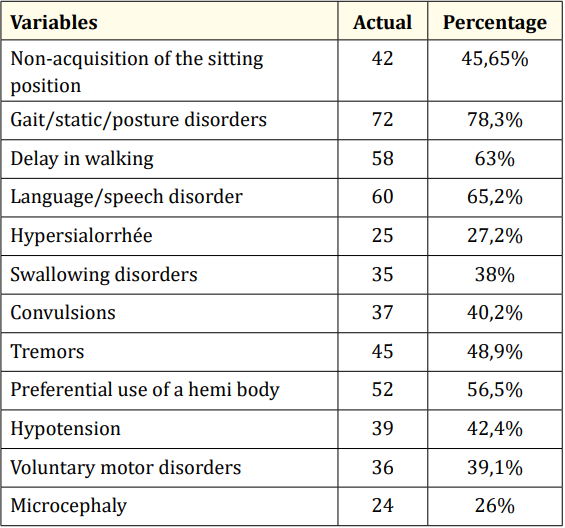
Table 2: Top Reasons for Consulting Children with CP.
Clinically, lA spastic tetraparesis was the form Predominant in 71.7% of cases (Figure 2).
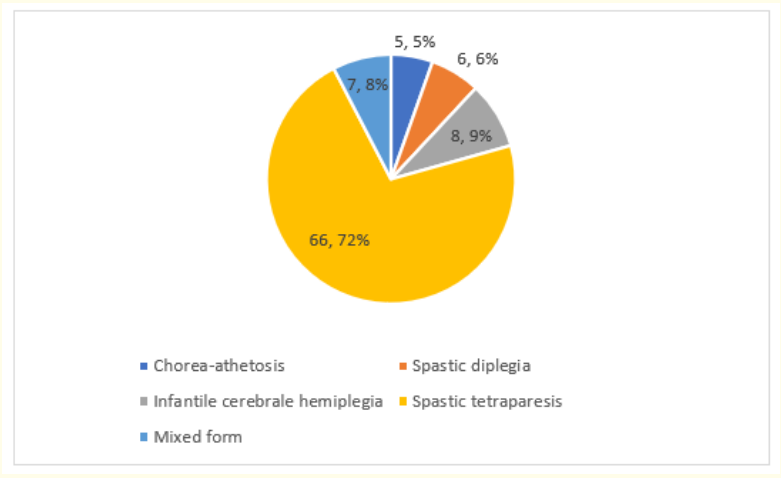
Figure 2: Clinical forms of cerebral palsy in our study.
We found osteoarticular deformities in 23.9% of patients (Table 3). The most frequent were the PBVE (31.8%), the flat foot valgus (27.3%) and the e genu flessum (18.2%).
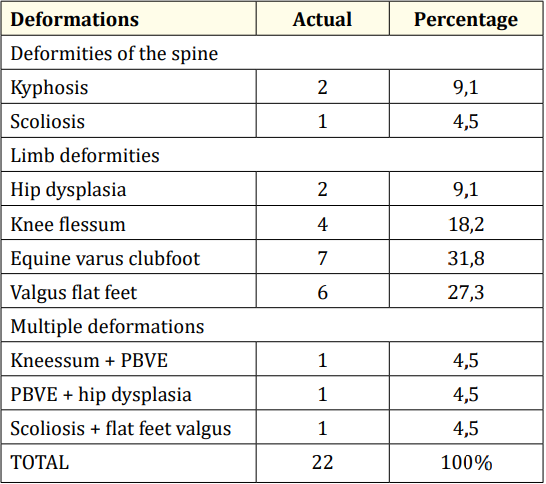
Table 3: Osteoarticular deformities observed in children with CP.
After assessment, 41.3% of children had a developmental quotient ( QD) between 20-34, reflecting severe delay (Figure 3).
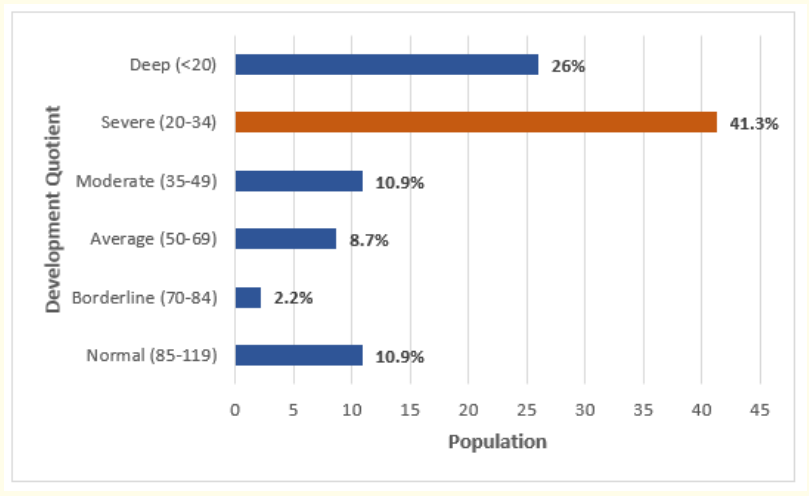
Figure 3: Distribution of patients according to their developmental quotient.
The main comorbidities most found in our series were oral facial dyspraxia (72.8%), epilepsy (70.7%) and language/speech disorders (65.2%).
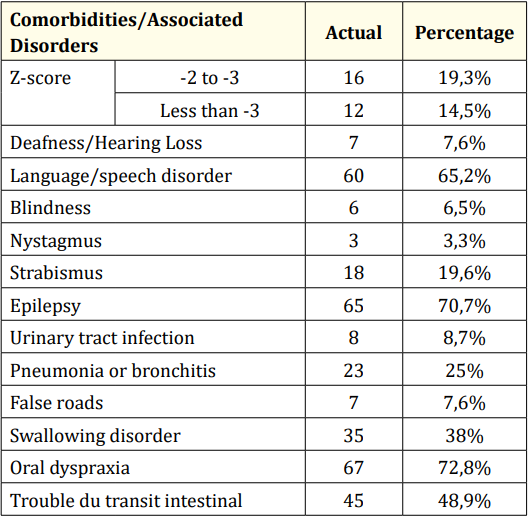
Table 4: Comorbidities and rubles associated with CP.
In terms of autonomy, nearly 2/3 of these children (64.6%) had a severe disability (IV and V) justifying the permanent use of a third person (Figure 4).
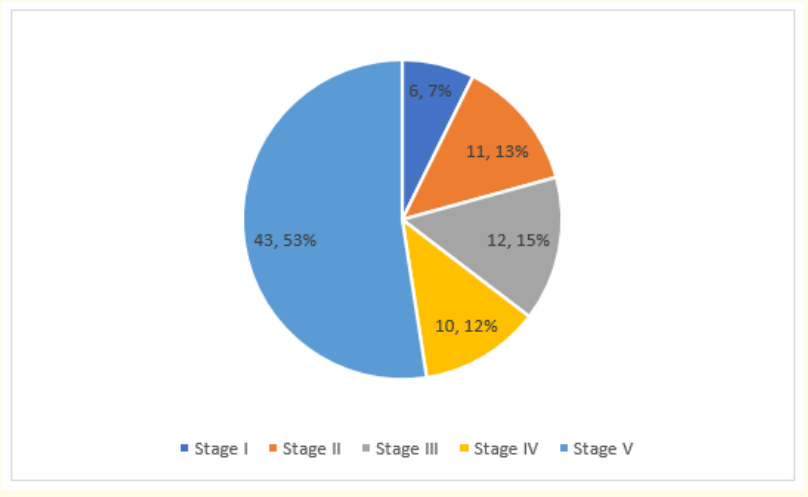
Figure 4: GMFCS Assessment of the Degree of Disability of Children with CP.
Regarding prognostic factors for severity, we have identified four: profound mental retardation, language disorders, transit disorders and irritability. Patients with severe disability were nine times more likely to have a QD ≤ 20 (OR = 9.58; p = 0.0006). It was fivefold for language disorders (OR = 4.56; p = 0.003), by three for transit disorder (OR = 3.13; p = 0.032) and by four for irritability (OR = 4.44; p = 0.008). There was no statistically significant association between severe disability and the etiology of CP.
The aim of our study was to describe the epidemiological, clinical and prognostic aspects of the severity of cerebral palsy in order to improve their management in our context.
We collected 92 children with CP with a hospital prevalence of 18.6% and a peak at ]1-5] years (4-6.7%). These results are close to thisux reported by Bearden., et al. [2] in Botswana and by Nguefack., et al. [6] in Cameroon where 85.1% of its population was under 5 years of age. This could be explained by the fact that clinical manifestations occur early on the one hand and on the other hand, the latest consultations are made around 3-5 years when the child reaches school age but is not there. obviously not fit.
CP preferentially affected boys with a sex ratio of 1.24. This predominance is corroborated by other authors [7,8], partly explained by the protective role of female sex hormones on the central nervous system in case of anoxia [8].
In our study, we identified several risk factors described in the literature, including vaginal birth (82.6%), neonatal seizures (46.7%) and maternal prenatal infections (39.1%), in decreasing order of frequency. Bass birth is a classic [9] but also home birth [10] and instrumental birth [10,11]. Several authors [5,10,12] have demonstrated a significant association between maternal infections during pregnancy and the occurrence of CP, probably due to impaired immune response in pregnant women. In addition, pre-eclampsia was found in 8% of the cases in our series. Maternal hypertension, which causes placental dysfunction, could compromise the fetus’ blood supply, cause growth retardation, hypoxaemia and irreversible brain damage. Some studies support this risk of CP in pre-eclampsia [11,13]. In our study, neonatal asphyxia was implicated in 48.9% of cases. While this correlation is shared by some authors [2,6,14], the causal link is questioned by others [12,15-17] that contrast improved perinatal care and declining perinatal mortality with stabilization of CP incidence over the past 2 decades. For these authors, the correlation would be below 10%.
In clinical forms, spastic tetraparesis was the most common (71.7%) in our population. This predominance is frequently reported in the literature [6,17-19].
The existence of osteoarticular deformities was common, affecting about 1 in 4 children (23.9%), mainly in the feet (54.5%) and knees (18.2%). On the other hand, the involvement of the spine (13.63%) was lower. O-skeletal muscul imbalances could be related to CP but also the sub-optimal management of these patients in particular, as Kakooza., et al. point out. [18] in resource-limited countries justifies its frequency. For Odding., et al. [17], hand sensitivity is impaired in 50% of cases and more than a quarter will present chronic pain in adulthood.
The main comorbidities most found in our series were oralfacial dyspraxia (72.8%), epilepsy (70.7%) and language/speech disorders (65.2%). Epilepsy in CP children is common, severe, and relatively refractory to medical treatment [17,20-22]. It would be major in spastic forms and in case of associated mental retardation [22]. This could explain this high prevalence in our series.
We found a statistically significant association between severe disability and language impairment (OR = 4.56; p = 0.003). Depressed Apgar score (50%), neonatal asphyxia (48.9%) and neonal seizures (46.7%) may be contributing factors in the occurrence of brain damage responsible for language. This correlation is documented by other authors [17,21,22].
On the other hand, patients who had a higher GMFCS were more likely to have severe to profound mental retardation (QD ≤ 34). In a study conducted by Bearden., et al. [2], 82% of patients had cognitive impairment, which was significantly common in patients with higher GMFCS.
In addition, PC children with severe disability were three times more likely to have a transit disorder, assessed at 48.9% in our series. This rate is superimposed on that of Odding., et al. [17] who found 50% gastrointestinal disorders. Indeed, the difficulties of selectivity and neuro-motor control to the pelvic floor, sphincter and abdominal muscles of PC children could justify these digestive disorders.
Our study found that CP is a deeply disabling condition in our context. From osteoarticular deformities to multiple comorbidities that complicate clinical expression, the condition of these PC children requires the development of optimal prevention strategies for the risk factors identified on the one hand and better screening for severity factors in our environment on the other. Further studies are needed to refine our knowledge of CPs and justify adapted therapeutic management algorithms.
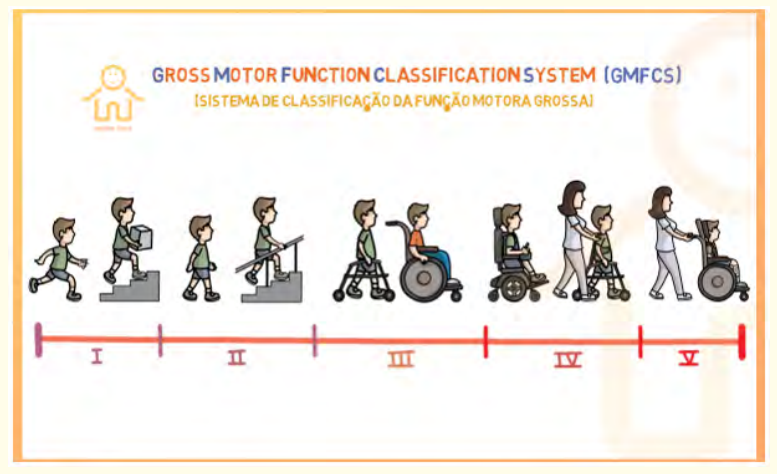
Annex 1: Gross Motor Function Classification System. 1GMFCS-E 8 R 2007 can child center for childhood Disability Research, Mc Master University (Dev Med child Neurol 1997;39: 214-223).
Copyright: © 2023 Ngo Yamben Marie-Ange C., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.