Yaser El Saba*, Muzammil Hafeez, Mustafa Bolkini, Niyas Ambadi and Moustafa Hassan
Department of NICU, Dubai Hospital, Dubai, UAE
*Corresponding Author: Yaser El Saba, Department of NICU, Dubai Hospital, Dubai, UAE.
Received: October 10, 2022; Published: November 30, 2022
Citation: Yaser El Saba., et al. “Rare Case of Spontaneous Fetal Massive Intracranial Bleed”. Acta Scientific Paediatrics 5.12 (2022): 32-36.
Fetal intracranial hemorrhage is a rare complication diagnosed during pregnancy with subsequent neonatal neurological morbidities or neonatal mortality. Intracranial hemorrhage is most commonly identified prenatally as intraventricular hemorrhage (IVH), although hemorrhage can occur in other sites such as cerebellar, subdural, primary subarachnoid and miscellaneous intraparenchymal hemorrhages. The detection of fetal intracranial hemorrhage has increased due to the improvement in imaging technology. There are varied etiologies of intracranial hemorrhage occurring prenatally, but in many cases, etiology remains unidentified. The outcome depends on the etiology, timing and the severity of fetal intracranial hemorrhage. Here we report a case of preterm baby born at 32 weeks who was diagnosed prenatally to have fetal intracranial hemorrhage with no obvious determined cause and managed by surgical evacuation.
Keywords: Spontaneous Fetal; Massive Intracranial Bleed; Etiology.
Intracranial Hemorrhage (ICH) refers to hemorrhage anywhere within the cranium. Although an estimate of 0, 5-1 in 1,000 pregnancies has been reported by referral centers, the incidence of ICH is unclear [1,2]. It is most commonly identified prenatally as IVH, although hemorrhage can occur in other sites such as cerebellar, subdural, primary subarachnoid and miscellaneous intraparenchymal hemorrhages. Recently, varieties of genetic mutation leading to abnormal connective tissue formation had been implicated in the genesis of antenatal ICH [3,4]. Spontaneous fetal ICHs may result from abnormal connective tissue architecture which globally affects fetal brain. Antenatal fetal intracranial hemorrhages may occur spontaneously or in association with various maternal or fetal conditions.
From the embryologic-pathological point of view, vascular connection between germinal matrix and the subependymal venous network, which are bleeding sites in IVH, are clearly present only after 20 weeks of gestation [5]. Therefore, fetal ICHs are usually deing tumors, anomalies, twins, and metabolic defects [5]. Strigini., et al. [6] showed that US had low diagnostic sensitivity in diagnosing small periventricular hemorrhage, and suggested that MRI evaluation should be a part of the diagnostic algorithm for hydrocephaly. Fetal MRI is a safe technique that provides high resolution of the fetal anatomy. In addition, MRI may be helpful for the estimation of the time of bleeding, and evolution of hematoma [7]. After birth, characteristic signal changes on T1-weighted and T2-weighted MRI sequences corresponding to blood product composition allow estimation of the timing of brain parenchymal hemorrhage [8].
Antenatal work-up to detect underlying etiology includes detailed history of drug exposure, abdominal trauma, history of previous births, assays for isoimmune and alloimmune thrombocytopenia, coagulation tests to exclude coagulopathies, and maternal serologic studies for cytomegalovirus, rubella, toxoplasmosis, parvovirus B19 infections. The evaluation should include family history of bleeding disorders, complete blood count, peripheral blood smear, coagulation tests (activated partial thromboplastin time, and prothrombin time), factor XIII levels, and bleeding time [9].
There are limited data on neurodevelopmental outcomes when fetal ICH is diagnosed antenatally. Ghi., et al. [10], in their extensive literature review of 93 cases, and 16 cases of their own, reported a generally poor prognosis of the newborn with antenatal diagnosis of ICH, with about 40% of fetus dying either in utero or within the first month after birth, and fewer than half of survivors being neurodevelopmentally normal at short-term follow-up. Similarly, Elchalal., et al. [1] reported nine fetuses with grades 3-4 IVH, all of which showed moderate to severe neurological deficits, one of which died at 2 months, in their series including 13 cases. Previous studies showed that, intrauterine progression of the extent of hemorrhage from a lower (Grades 1 and 2) to a higher grade is associated with a worse neurodevelopmental prognosis. In contrast, complete resolution of sonographic findings is not uncommon in Grade 1-2 lesions, and the prognosis are usually excellent [1,7,10].
A male baby born preterm, at 32 weeks of gestation to a 34-year-old G8P5A2 mother. Her antenatal scan showed umbilical vein varix measuring 0.8 cm. Ultrasound scan done in fetal medicine unit showed fetal brain having subcortical hemorrhage and clot formation at the left cortical area. Fetal MRI revealed bilateral large subacute subdural hematoma with minimal interhemispheric fissure extension causing moderate extra-axial mass effect on cerebral cortex (Figure 1).
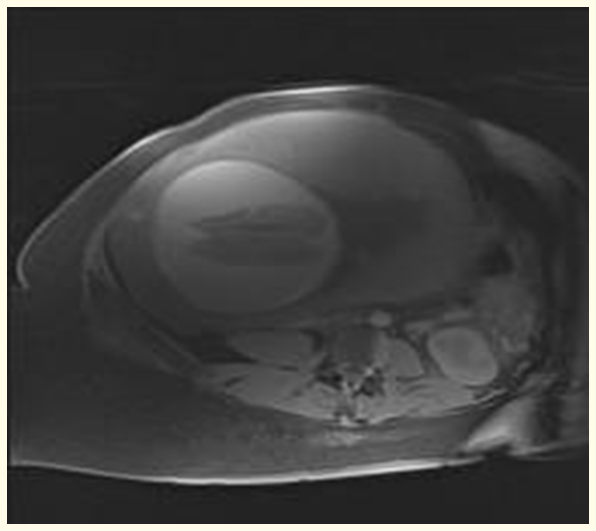
Figure 1: Feta MRI T1 image showing bilateral large subacute subdural hematoma.
Her antenatal period was otherwise unremarkable. There is no history of trauma during her pregnancy. Her routine lab studies were normal. Her HIV, HBsAg, TORCH screening and HVS culture for GBS was negative. She was not on any medications like aspirin or warfarin.
Baby delivered by emergency LSCS in view of fetal distress and required resuscitation as baby was apneic; he improved with IPPV and kept on NPIMV support. Apgar score was 6 and 9 at 1 and 5 minutes respectively. He was weaned off from ventilator within next 24hours.
He weighed 2400g (50th centile) with length 41 cm (>10th centile) and head circumference 38 cm (>97th centile on Fenton preterm chart).
He was markedly pale and had tense and bulging anterior fontanelle with widely separated sutures. The rest of his physical examination was normal. He did not have any convulsions throughout the NICU stay and was clinically stable without any neurological deficits. Ophthalmological examination was normal.
Lab investigations showed Hb of 5.3gm/dl, PT >120 s and PTT >160 s. This was corrected with transfusions of Packed Red blood cell and Fresh frozen plasma along with Vitamin K.
His FBC (except for HB), peripheral smear and bleeding time was normal. Coagulation factors levels of factor V, VII, VIII, IX and XIII were not conclusive of any factor deficiency. Von Willebrand’s factor was normal. TORCH, Thrombophilia and metabolic screening was also normal.
Brain MRI revealed subdural, intracerebral, intracerebellar and intraventricular hemorrhages with supratentorial hydrocephalus (Figure 2). As the baby was clinically, stable neurosurgical intervention was planned for a later date after further imaging.
FBC, PT PTT, Coagulation factor levels including factor 13 were repeated after 1 month which was normal. Abdominal ultrasound scan, Brain MRA and MRV was normal.
Repeat Brain MRI at 1 month of age showed progressive course as regard to the size of the left sided fronto-parieto-occipital extra axial collection with a thickness of 3.4 cm compared to 2.6 cm in the previous study associated with considerable mass effect in the form of contralateral midline shift of about 9.5 mm and effacement of the left lateral ventricle (Figure 3). Regression of the right sided fronto-parieto-occipital extra axial collection was also noted. Asymmetrical dilatation of the ventricular system was more evident on the right side.
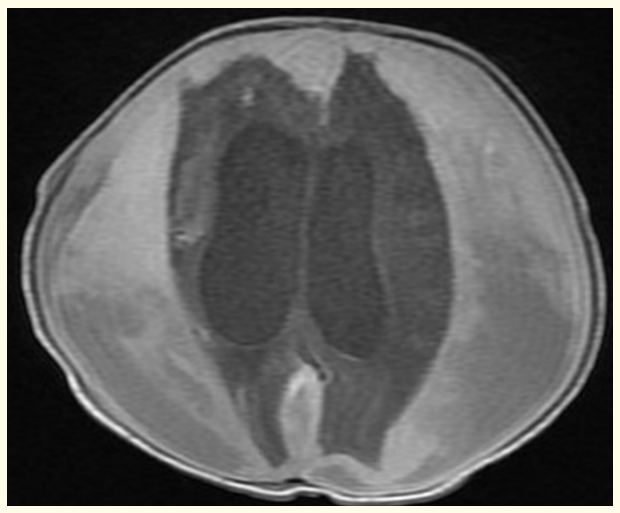
Figure 2: Postnatal MRI brain T1 image showing subdural, intracerebral, intracerberral and intraventricular hemorrhages with supratentorial hydrocephalus.
Bur hole drainage of the intracranial collection was done by the neurosurgeon and drained about 30 ml of old blood. His head circumference had decreased by 2cms. He was active and feeding well. On examination there was no neurological deficits and was discharged home.
On review by the Pediatric neurologist and neurosurgeon, at corrected age of 4 months, he is active feeding well and gaining adequate weight. There is no history of any convulsions.
His head circumference is 40 cms. (9th centile on Fenton chart). He is alert, focusing and following objects. Hearing, cranial nerves examination is normal. Post-operative Ct scan brain showed significant interval regression of the bilateral subdural hematoma with no significant compression upon the underlying cerebral cortical sulci and persistent asymmetrical dilatation of the lateral ventricles and mild dilatation of the third ventricle (Figure 4). Pupils bilaterally equal and reactive to light. Extra ocular movements are normal and there is no nystagmus. His deep tendon reflexes are abnormal in the form of exaggerated knee reflex and non-sustained ankle clonus. His limb movements are normal. Except for mild developmental delay, there is no obvious neurological deficits noted. He is planned for serial follow up in clinic and for follow up imaging of the brain.
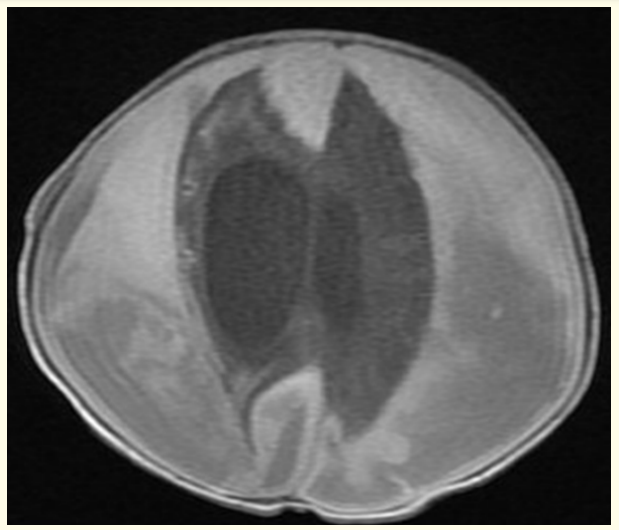
Figure 3: Postnatal MRI brain scan T1 image at one month of age showed progressive course as regard to the size of the left sided fronto-parieto-occipital extra axial collection.
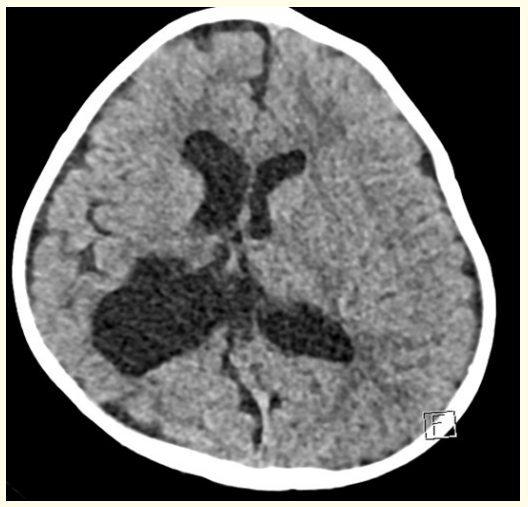
Figure 4: Post-operative CT scan brain showed significant regression of bilateral subdural hematoma with mild asymmetrical dilatation of the lateral ventricles.
Intracranial hemorrhage (ICH) is a common complication of premature infants and may occur in utero [11].
ICH is most commonly identified prenatally as intraventricular hemorrhage (IVH), although hemorrhage can occur in other sites such as cerebellar, subdural, primary subarachnoid and miscellaneous intraparenchymal hemorrhages [12].
Although an estimate of 0, 5-1 in 1,000 pregnancies has been reported by referral centers, the incidence of ICH is unclear [1,2].
In our baby, he had fetal intracranial hemorrhage as evidenced by the brain ultrasound scan which showed fetal brain having subcortical hemorrhage and clot formation at the left cortical area and fetal brain MRI which showed bilateral large subacute subdural hematoma with minimal interhemispheric fissure extension causing moderate extra-axial mass effect on cerebral cortex.
Fetal intracranial hemorrhages may occur spontaneously or in association with various maternal or fetal conditions.
Predisposing maternal conditions include platelet and coagulation disorders, medications (warfarin or illicit drugs like cocaine), maternal seizure, smoking, trauma, amniocentesis, and febrile disease [3,4,13-16].
Predisposing fetal conditions include twin-twin transfusion, demise of a co-twin, hydrops fetalis, congenital tumors, and fetomaternal hemorrhage [6,17-19]. The most common risk factor for intracranial hemorrhage is a platelet disorder. Intrauterine demise of a monochorionic twin may result in severe ischemic injury and extensive ICH in the surviving twin [20]. Maternal infections can seem mild yet have significant consequences for the fetus. An example of this is maternal primary cytomegalovirus infection which can cause fetal intracranial bleeding as well as the more frequently encountered migrational disorders, brain destruction and calcification. [12]. Recent work has identified genetic mutations that may predispose to ICH [5]. In many cases however, the cause is not identified [1]. In the present case there were no maternal or fetal risk factors, except that the antenatal ultrasound scan showed umbilical vein varix measuring 0.8 cm.
Our baby after birth was active but pale with a hemoglobin of 5.3g/dl. His anterior fontanelle was bulging. Ultrasound scan of the brain showed large bilateral subdural haematomas compressing the brain parenchyma, with hydrocephalus. MRI of the brain showed large bilateral subdural haematomas compressing the brain. Multiple parenchymal haemorrhages in both cerebellar lobes in addition to right frontal and left occipital lobe with intraventricular haemorrhages and supra tentorial hydrocephalous.
Baby was extensively investigated for the cause of the intra cranial hemorrhage. His initial Prothrombin time was prolonged (more than 120 seconds) and his Partial thromboplastin was also prolonged (more than 160 seconds). This improved with one fresh frozen plasma transfusion and Vitamin K injection. His bleeding time, clotting factor assay were normal and cause for the bleeding could not be found.
He was initially conservatively managed after the opinion of the neurosurgeon. He did not have any altered tone or convulsions. Brain MRI at 1 month 10 day of age showed Progressive course as regard the size of the left sided fronto-parieto-occipital extra axial collection and also a mid-line shift. The subdural hematoma on the left was evacuated through the burr hole.
On follow up in the outpatient clinic, the baby seen by the neurosurgeon and neurologist. He is having exaggerated knee and ankle reflexes, mild developmental delay. His cranial nerves, limb movements hearing is normal. His repeat ultra sound scan showed Moderate dilatation of the supra tentorial right lateral ventricle, there is no intra or extra axial collection.
In conclusion, fetal high grade and progressive ICH carries a significant risk of postnatal neurodevelopmental impairment. The early detection of these fetuses by US, and fetal MRI is very important to provide appropriate counseling to the families. However, the management options are mainly based on timely termination of pregnancy in severe cases, and conservative approaches including early postnatal neurological evaluation, shunting operation or evacuation, and implementing special rehabilitation program in milder cases.
The authors of this paper declare no conflict of interest.
Copyright: © 2022 Yaser El Saba., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.