Adriana Costa*, Sara Completo and Paula Correia
Department of Child and Youth, Hospital Professor Doutor Frenando Fonseca, EPE, Portugal
*Corresponding Author: Adriana Costa, Department of Child and Youth, Hospital Professor Doutor Frenando Fonseca, EPE, Portugal.
Received: August 24, 2022; Published: November 15, 2022
Citation: Adriana Costa., et al. “Invasive Disease in Pediatric Population - 10 Year Review”. Acta Scientific Paediatrics 5.12 (2022): 10-18.
Introduction: Pediatric invasive disease (PID) is confirmed by isolation of a microorganism from a normally sterile site. The incidence of infection due to multi-drug resistant (MDR) bacteria is increasing worldwide, which threatens the effective treatment of infections. This study aims to characterize PID during 10-years in a Portuguese hospital: morbimortality, risk factors and antimicrobial susceptibility.
Methods: Descriptive retrospective study of PID in children aged between 28 days and 18 years old, attended in a pediatric department from 2010 to 2020.
Results: 236 PID episodes were identified among 230 patients. Comorbidities were present in 25.4%, predominantly prematurity (37.7%) and sickle cell disease (30.4%). The median age was 21.4-month-old. The most common diagnosis was bacteremia without a source (34%) and pneumonia (18%). Sepsis occurred in 17.4%. Clinical sequelae were present in 17.4%. Pneumonia and meningitis were associated to worse outcomes (p-value < 0.001). Mortality rate was 1.3%. Were isolated 279 microorganisms: 88% in blood; 6% in cerebrospinal fluid; 4% in pleural liquid; 2% in joint fluid. The most frequently isolated were Streptococcus pneumoniae (22.6%) and methicillin-susceptible Staphylococcus aureus (13.3%); 7.5% were MDR bacteria, of which 33% were community-associated.;
Conclusion: PID is still an important cause of morbimortality. The incidence of MDR microorganisms is alarming, which alerts to the problem of wide use of antibiotics, especially broad spectrum.
Keywords: Bacteria; Fungi; Infectious Disease; Multi-Drug Resistance; Antimicrobial Susceptibility.
MDR: Multi-Drug Resistant; MSSA: Methicillin-Susceptible Staphylococcus Aureus; PID: Pediatric Invasive Disease
Invasive pediatric disease (PID) is a clinically compatible case confirmed by isolation of a microorganism from a normally sterile site. It can be caused by many microorganisms, including bacteria and fungi. Streptococcus pneumoniae is the most common agent responsible for severe bacterial infections, such as bacteremia (affecting mostly children from six to 36 months), meningitis (mostly children from six to 18 months) and osteoarticular infections (mostly children from three to 34 months) [1,2]. Fungal disease is also an important part of PID, especially in immunocompromised patients [3].
Children under five years of age, and specially under two years of age, are more susceptible to PID, partially due to the immature immune system and, not only frequent exposures to infection but also colonization by S. pneumoniae [2].
The introduction of routine immunizations has reduced the incidence of PID. Nevertheless, it is, still, an important cause of morbidity and mortality. Wide vaccination is thought to have changed the serotypes causing PID, mostly for nonvaccine serotypes. Also, the larger use of antimicrobials has been causing an increase in multi-drug resistant (MDR) microorganisms, hampering treatment [1,2,4].
Risk factors can vary depending on the microorganism. General risk factors for PID include: younger age (less than five years), asplenia (functional or anatomical, including sickle cell disease), human immunodeficiency virus infection, immunoglobulin deficits, aplastic anemias, hematopoietic cell transplant, malignancy (such as acute myeloid leukemia and acute lymphoblastic leukemia) and associated underlying condition, namely neutropenia and immunosuppressive therapies [5,6].
The problem of antimicrobial resistance is one of the foremost issues that we will face in the coming decades. The incidence of infection and colonization due to MDR bacteria is increasing in hospitals worldwide. For MDR bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), there are some well-known risk factors: prior antibiotic use (in the last six months), previous infection or colonization with these microorganisms; hospitalization lasting more than 48 hours in the last six months (especially if it was prolonged and in intensive care units); surgery or dialysis; wound care; presence of indwelling medical devices such as urinary catheters, feeding tubes, endotracheal tubes and vascular lines [7,8].
Diagnosis of PID is usually difficult, as it is essentially made by direct observation of the sterile products, often obtained through invasive procedures, which results in a low sensitivity for microorganism identification. Nowadays there are Polymerase Chain Reaction techniques, which make diagnosis somewhat easier [3].
Early suspicion and subsequent treatment are crucial for reducing complications. However, despite aggressive approach, the only way to reduce PID related mortality, is to prevent the disease [4].
The aim of this study was to characterize PID, namely the diagnoses associated with invasive disease, its morbidity and mortality, associated risk factors and the pattern of resistance to antimicrobials in a frame period of 10 years in the pediatric department of a level II hospital in Portugal.
This was a retrospective study of PID in patients with age comprised between 28 days and 17 years and 365 days old. We analyzed data from 1 January 2010 to 31 December 2019 of PID cases in children observed in a level II Portuguese hospital in the surroundings of Lisbon, that treated an average of 1790 pediatric patients annually, in the past 10 years.
Pediatric invasive disease was defined as a clinically compatible case confirmed by isolation of a bacteria or fungi from a normally sterile site (blood, cerebrospinal fluid, joint fluid, pleural fluid or pericardial fluid). Only agents isolated from samples of these sterile body fluids were considered. The various specimens were processed in the microbiology laboratory by standard techniques according to our hospital protocol.
Demographic characteristics (age and sex) and clinical information (underlying diseases, site of isolation and results of antibiotic susceptibility testing, outcome and mortality) were obtained from clinical records.
Were excluded from this study children treated in the neonatal intensive care unit, bacteria or fungi isolated in cultures of nonsterile products and contaminated blood cultures.
Infections can be divided into community onset and nosocomial acquisition. In this study we used the most frequent cut-off to distinguish between these two categories: whether the onset of infection was within the first 48 hours of hospitalization (communityonset) or later (nosocomial) [8].
Statistical analysis was performed with SPSS Statistics version 25 (SPSS Inc, Chicago, IL, USA). The significance level was set on 0.01.
The study was approved by the ethics committee of our hospital. All information was anonymous and confidential.
We analyzed data of PID in a pediatric population for a period of 10 years, with a total number of 230 patients, in which 60% were male. Ninety percent were Portuguese and 7% were from African countries (Table 1).
There were 236 episodes of invasive disease. The average number of PID cases per year was 24 (minimum 18; maximum 31). The median age of children by episode was 21.4-month-old (interquartile range 8.1-70.3 months). At least one comorbidity was present in 25.4% of the invasive disease episodes, the most frequent being prematurity (37.7%), followed by sickle cell disease (30.4%), chronic lung disease (30.4%), immunosuppression (13%) and malignancy (2.9%) - (Table 1).
Hospitalization was required in 206 episodes. During the period of our study, there were a total of 16139 hospitalizations by any motive in children older than 28 days of age. PID represented 1.3% of the total of hospitalizations, which accounts for an incidence of 13 cases per 1000 hospitalized children. As the number of annual cases has not changed much over the years, the disease incidence evolution curve is relatively stable (Graphic 1).
The most frequent diagnosis was bacteremia without a source (33.8%), followed by pneumonia (17.6%), meningitis (10.4%) and urinary tract infection (8.4%) - (Table 2). Considering diagnoses per age group, in all groups the most frequent was bacteremia without a source, except for children aged between 36-month-old to nine years-old, in which the most frequent diagnosis was pneumonia (Table 3).
There were identified 279 microorganisms in microbiologic cultures, corresponding to 269 bacteria and 10 fungi. The isolates were 88% in blood, 6% in cerebrospinal fluid, 4% in pleural liquid and 2% in joint fluid. The most common isolated microorganism was Streptococcus pneumoniae in 22.6%, followed by MethicillinSusceptible Staphylococcus aureus (MSSA) (13.3%) and Escherichia coli (10.4%) - (Table 4).
We analyzed the microorganisms responsible for PID by age group. The most common microorganism causing PID in children between one and two months old was Enterococcus faecalis (6/27; 22.2%). S.pneumoniae was the most frequent in the next two age groups, which include children aged three to 35 month-old (38/150; 25.3%) and 36 months to nine years-old (8/58; 31%). Concerning children over the age of 10, E. coli and MSSA were the two most frequently identified microorganisms (8/44; 18% each).
Regarding Haemophilus influenzae infection, we reported five cases identified in blood cultures: three unecapsulated H. influenzae and in two cases it was not possible to identify the H. influenzae serotype. Three of these cases occurred in children previously vaccinated against H. influenzae type b.
Regarding the 12 cases of Neisseria Meningitidis infections (eight identified in blood and four in liquor), 60% occurred in children aged four to six months. Nine corresponded to serogroup B, two to serogroup Y and in the other case it was not possible to identify the serogroup. All of these children were not vaccinated against the N. meningitidis serogroup responsible for the infection.
Concerning the 63 cases of S. pneumoniae infections, 49 were identified in blood, nine in liquor and five in pleural liquid. We evaluated the vaccination status of children with invasive pneumococcal disease. Three cases fulfilled the criteria of vaccine failure in which invasive disease occurred in a fully vaccinated child without immunossupression or immunodeficiency: one failure of 7-valent pneumococcal conjugate vaccine (serotype 4 isolation) and two cases of 13-valent pneumococcal conjugate vaccine failure (PCV13 - serotypes 19A and 6B). One child was considered to be incompletely vaccinated before PID (2 doses of PCV13 under 12 month of age), with identification of a serotype 6B. No underlying comorbidities were identified in these cases.
In our study 20% (56/279) of the infections were nosocomial (Table 4). A central venous catheter was present in 77% of the previously mentioned cases (43 of 56 cases).
Regarding the analysis of antibiotic resistance in specific agents: 100% of the identified Streptococcus pneumoniae had full susceptibility to penicillin and to ceftriaxone; 17.2% (5/29) of the nonextended-spectrum β-lactamase-producing (ESBL) E. coli showed resistance to amoxicillin/clavulanic acid and all of them were susceptible to cefuroxime.
We identified a total of 21 MDR bacteria (7.5% of the isolates), being 33% of these responsible for community-associated infections. At least one known MDR risk factor was present in 71.4% of cases, while 9.5% did not present any risk factor and data was not available in 19% of the cases. Regarding the identified risk factors, the most frequent were recent antibiotic therapy (61.9%) or hospitalization in the last six month (61.9%). A previous MDR infection was documented in 19% and 33% were carriers of indwelling medical devices.
The most common MDR was ESBL Enterobacteriaceae with 57% of MDR positive cultures (Table 5).
A total of 10 isolations of fungi in blood culture were identified (3.6% of the total isolated microorganisms). The incidence of invasive fungal disease in our population was 6 cases per 10000 hospitalized children. Seven of them were isolated in the context of nosocomial infection, in patients with at least one risk factor, namely prematurity, immunodeficiency, sickle cell disease or presence of central venous catheter. Of the three non-nosocomial fungi identified in blood cultures, only one of them, corresponding to a Candida sp., was isolated in a 12-month-old child without any risk factor; the other two corresponded to a child with sickle cell disease and another with prematurity.
Regarding clinical evolution, in 29% (68/236) of episodes, a central venous catheter was placed. Sepsis occurred in 17.4%. There was a total of three deaths due to PID, which corresponds to a case fatality rate of 1.3%. The deaths correspond to: a 2-year-old patient with sickle cell anemia that developed a Streptococcus pneumoniae sepsis; a 2-month-old premature child with pulmonary chronic disease that had a pneumonia with isolation of Enterococcus faecalis in blood and pleural liquid; a 2-year-old patient with a septic shock in which it was isolated an Enterococcus faecalis and Streptococcus pyogenes in blood cultures.
At least one clinical sequela was present in 17.4% (41/236). Applying Pearson’s chi-squared test, there is a significant correlation between the diagnosis and the clinical evolution (p-value < 0.001). Pneumonia and meningitidis were the diagnoses associated to worse outcomes. Pneumonia was associated with 34.1% of the registered sequelae and meningitidis was responsible for 29.3%. There were limited data regarding the characterization of these sequelae and their long-term evolution.
Table 1: Characterization of the population with Invasive Pediatric Disease between 2010-2020 in a Portuguese Pediatric Hospital.
Table 2: Diagnoses associated to Invasive Pediatric Disease.
Table 3: Distribution of the most frequent diagnosis and microorganisms per age group.
Table 4: Isolation of microorganism in patients with PID - nosocomial and community infections.
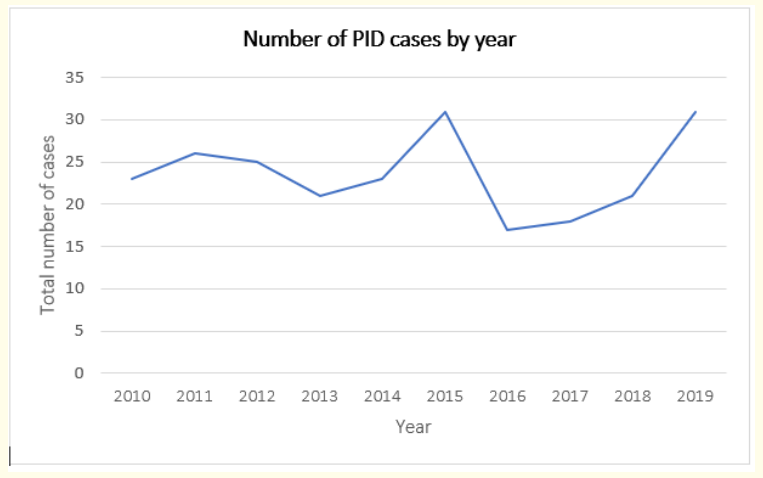
Graph 1: Evolution of the number of pediatric invasive disease cases by year between 2010 and 2020.
In this study, the authors describe the clinical characteristics of a large series of cases of invasive disease in children over a 10-year period in a level two hospital in Portugal. We may confirm that PID is still prevalent in our population, in a rate of 13 per 1000 hospitalized children, and also an important cause of morbidity and even mortality, besides the great effort to implement vaccination and other preventive measures. We emphasize that 88% of these children needed hospitalization, which has an important psychological, social and economic impact.
It is well recognized that children with underlying conditions remain at increased risk of infectious disease and more severe clinic and evolution [2,5]. In our data, at least one comorbidity was present in 25.4% of the children.
Multidrug resistant bacteria are well-recognized to be one of the most important current public health problems worldwide. Typically, MDR bacteria are associated with nosocomial infections. However, some MDR bacteria have become quite prevalent as causes of community-acquired infections [8]. In our hospital, during the period considered, 7.5% of the microorganisms identified corresponded to MDR bacteria, being 33% of these responsible for community-associated MDR infections. We further emphasize that most children with MDR infections had either received antibiotic therapy or been hospitalized in the previous six months. This data alerts to the problem of wide use of antimicrobial treatment. It is important to highlight that only the isolations that conditioned the presence of invasive disease were presented. Therefore, the incidence of MDR infection or colonization should be much higher.
Regarding antimicrobials susceptibility, we underline that, in our sample, all the S. pneumoniae were susceptible to penicillin, which is different from what has been reported in other studies that observed higher nonsusceptibility rates to penicillin in isolates from children [9]. Of note, 100% of non-ESBL E. Coli were susceptible to cefuroxime, which is in agreement with many studies that show high susceptibility profiles to this antibiotic [10].
Taking into account the reported incidence of invasive fungal disease of six cases per 10 000 hospitalized children, we may also conclude that invasive fungal disease is rare in children, as it was already reported in other previous studies [11].
Many studies report meningitis and pneumonia as life-threatening diseases, often related with serious complications and sequelae [12,13]. Our study is in agreement with previous data, showing that these two diagnoses were the most associated with the presence of sequelae at the time of hospital discharge.
Some of the cases identified were vaccine preventable diseases. The Portuguese vaccination program has changed over the past 10 years, which might have had an impact on the results of this study, being one of the limitations we report. Highlighting the introduction of the PCV13 in the universal Portuguese vaccination program in 2015 (PCV13 and 23-valent pneumococcal polysaccharide vaccine, PPSV23, were administered with no costs only in risk groups, since 2010) and the free immunization with anti-Meningococcal Group B Vaccine in risk groups since 2016 extended to all children in 2020.
It is notable that PID is still an important cause of morbidity and mortality in children. Therefore, it is of major importance that PID is early suspected, correctly diagnosed and managed, so that we can reduce its prevalence and posterior sequelae. Nonetheless, more studies are needed to develop the knowledge about this thematic and improve health outcomes.
We thank Dr. Clara Portugal (Clinical Pathology laboratory - Directed by Dr. Luisa Sancho) for their assistance in data acquisition.
The Author(s) declare (s) that there is no conflict of interest; This research has not received specific help from public agencies, commercial or other non-profit entities.
Copyright: © 2022 Adriana Costa., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.