Mamadou Bountogo1,2*, Eric Nebié1,5,7, S Pascal Zabré1, Seraphin Simboro1, Ali Sié1,3,4, Nicolas Meda2 and Edouard Betsem6
1 Centre de Recherche en Santé de Nouna, Nouna, Burkina Faso, Africa
2 Université Joseph Ki-Zerbo, Ouagadougou, Burkina Faso, Africa
3 Heidelberg University, Heidelberg, Germany
4 Proctor Foundation, University of California San Francisco, San Francisco, USA
5 Swiss Tropical and Public Health Institute, Allschwil, Switzerland
6 Faculty of Medicine and Biomedical Sciences, Yaounde, Cameroun
7 University of Basel, Switzerland
*Corresponding Author: Mamadou Bountogo, Centre de Recherche en Santé de Nouna, Nouna, Burkina Faso, Africa.
Received: August 04, 2022; Published: August 16, 2022
Citation: Mamadou Bountogo., et al. “Trend in Childhood Mortality After Pneumococcal Conjugate Vaccine (PCV-13) and Rotavirus Vaccines Introduction in the Nouna Health and Demographic Surveillance System, Burkina Faso”. Acta Scientific Paediatrics 5.9 (2022): 15-21.
Background: Pneumonia and diarrhea remain two leading causes of children under 5 years mortality. PCV-13 vaccine and rotavirus vaccine were introduced into the Burkina Faso EPI in 2013. Given the diversity of circulating serotypes and vaccine schedules, the WHO recommended an evaluation of their actual effectiveness after their introduction in different countries. This study, using an interrupted time series analysis, proposes to evaluate the effectiveness of PCV-13 and rotavirus vaccine on under 5 years mortality.
Methods: The study used interrupted time series designs with a pre-intervention period from 2009-2013 and a post-intervention period from 2014 to 2015.The monthly mortality rate ratio was the variable of interest. A generalized linear model was applied using log-transformed mortality rates as outcomes, with a Poisson distribution, adjusted.
Results: The infant mortality rate ratio was 0.84 (95% CI = 0.58-1.22, p = 0.37) 0.90 (95% CI = 0.70-1.15, p = 0.43), 0.84 (95% CI = 0.68;1.02, p = 0.09) on children aged 0-59 months, 0-11 months, 12-59 months, respectively. Although not statistically significant, the results in this study showed a 16% decrease in mortality in children aged 0-59 months.
Conclusions: A positive effect of the newly introduced rotavirus and 13-valent pneumococcal conjugated vaccine was observed in the current study and needs to be consolidated in a bigger population size.
Keywords: Childhood Mortality; Trend; Nouna HDSS; PCV-13; Rotavirus Vaccines.
EPI: Expanded Program on Immunization; HDSS: Heath and Demographic Surveillance System; PCV: Pneumococcal Conjugate Vaccine; WHO: World Health Organization
Diarrhea and pneumonia are the two leading causes of children of post-neonate mortality in 2019 worldwide. Annual analysis of global mortality data from 2000 to 2019 shows that sub-Saharan Africa and Southeast Asia remain the largest contributors to under-five deaths [1] For this reason, the World Health Organization (WHO) and the Global Alliance for Vaccines and Immunization (GAVI) have encouraged and supported the introduction of pneumococcal conjugate and rotavirus vaccines in national expanded programs on immunization (EPI) in resource limited countries [2,3]. Several clinical trials have shown that pneumococcal conjugate vaccines are 12-50% effective in preventing invasive pneumococcal diseases caused by vaccine preventable serotypes [4-7]. Conjugate vaccines have effect on carriage of vaccine strains in vaccinated individuals breaking the human-to-human chain of transmission [8-10]. This protection known as herd or collective immunity depicts a more enhanced effect of the conjugate pneumococcal vaccine in countries with limited resources such as Burkina Faso. The replacement of circulating serotypes with emerging non-vaccine serotypes thus contributes to the reduction of the potential benefits of this vaccination [11,12]. Rotavirus is the leading cause of severe infectious diarrhea in children in developing countries [13-15]. Effective rotavirus vaccines have been developed in recent years and introduced into national expanded programs of immunization in SSA [16,17].
Rotavirus and conjugated pneumococcal vaccines were introduced in Burkina Faso late December 2013. Both vaccines are administered concurrently with pentavalent vaccine at 2, 3, and 4 months of life. The WHO recommended a post introduction assessment of these vaccines’ schedules and effectiveness on circulating serotypes [18,19]. Most of the impact studies carried out are hospital-based data [20,21]. In low incomes countries, more than 75% of deaths occur in the community and are not documented by health services according to Nouna HDSS 2010 data [22]. The current impact assessment of PCV and rotavirus vaccines in children under five years mortality in a rural population and health surveillance system in Burkina Faso aims to bridge this gap.
This study was carried out in the Nouna Health, and Demographic Surveillance System (HDSS) operated by the Centre de Recherche en Santé de Nouna (CRSN) in rural Burkina Faso (Figure 1). The population is approximately 100,000 inhabitants in 15 000 households across 58 villages and the town of Nouna in 2020. The CRSN conducts longitudinal monitoring of vital events as well as cross-sectional surveys in households [23]. During households’ visits, the interviewers regularly update the demographic data and vital events (death, birth…) that have occurred since the previous visit.

Figure 1: Nouna HDSS map.
The ITS was used in our study to evaluate the both the two vaccines (PCV-13 and rotavirus vaccine) effectiveness on child mortality in Nouna-HDSS. The ITS is a precious study design for evaluating the effectiveness of population-level health interventions [24]. ITS, sometimes called quasi-experimental time series analysis, is a method of statistical analysis involving the tracking of a long-term period before and after an intervention point to assess the effects of the intervention.
Children aged 0 to 59 months who are permanent residents of the Nouna HDSS were enrolled in this study.
In this study, the two variables of interest are death (dependent variable) and the introduction of PCV-13 and rotavirus vaccines. Verbal autopsies which are methods of determining causes of deaths is used to diagnose the causes of death using the primary data collected on signs and symptom of the deceased persons. The Inter-VA was used to determine the causes of death. This software has shown low sensitivity to determining infectious causes such as pneumonia and diarrhea [25-27]. That is why we only looked at allcause mortality in this study. For the introduction of the two vaccines, the variable is set to 1 after 2013 and 0 from 2009 to 2013.
The database with demographic was transformed according to the longitudinal procedures of INDEPTH [28,29] to allow a time series analysis. The variable date of the events was very important to allow the periodization in months to years for the calculation of the different indicators. Monthly mortality counts and mortality rates were used as the outcomes of interest. The analysis was initially descriptive and a measure of the pre- and post-introduction trends of the two vaccines was carried out. The pre-introduction period ranged from 1 January 2009 through 31 December 2013 and the post-introduction period ranged from January 1st, 2014 through December 31st, 2015. We did a segmented regression to model the mortality rate. The monthly mortality rate was converted to logarithm and an adjusted Poisson model was used considering overdispersion and seasonality.
We applied a generalized linear model using logarithm transformed mortality rates as results, with a Poisson distribution, adjusted accordingly to account for dispersion. The Breusch-Godfrey autocorrelation test was used. The efficacy of the two vaccines on infant and juvenile mortality was calculated as the mortality rate ratio (MRR) from the model. All statistical analyses were performed using STATA software (version 14). The significance threshold of 5% was considered.
During the 6-year study period, the Nouna-HDSS recorded 1954 deaths in under five-year children, of which 1358 (2009 to 2013) occurred before vaccines introduction in the Expanded Program on Immunization (EPI) and 596 from 2014 to 2015 after vaccines introduction in EPI. The lowest number of deaths was recorded in the month of June (n = 84 over 6 years) and the highest in August (n = 288 over 6 years). The year 2009 and 2013 recorded the lowest number of death while 2012 recorded the highest number followed by 2015. Deaths in children under one year accounted for 25% of all deaths in children under 5 years (Table 1).
Table 1: Under five-year death repartition in Nouna-HDSS from 2009 to 2015, Burkina Faso.
The infant-juvenile mortality ratio was 0.84 (95% CI = 0.581.22, p = 0.37) in children 0-59 months of age. The mortality ratio for children under 1 year of age and was 0.90 (95%CI = 0.70-1.15, p = 43) and those aged 12-59 months 0.84 (95%CI = 0.68-1.02, p = 0.09) (Table 2 and Figure 2).
Table 2: Efficacy of the introduction of the two vaccines (VPC13
and rotavirus) on under five-year children mortality, 2009-2015,
in Burkina Faso.
MRR* mortality rate ratio.
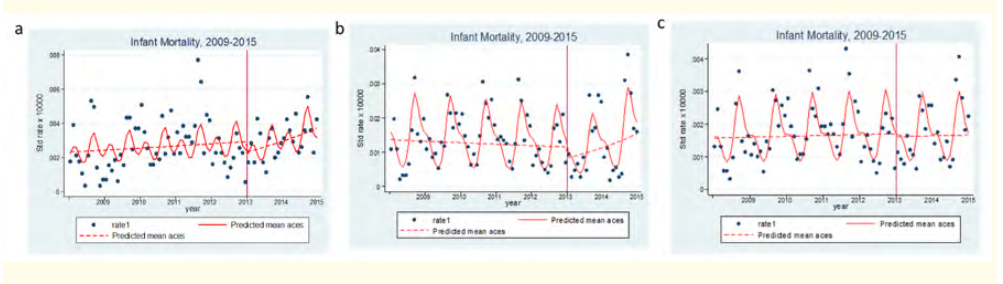
Figure 2: Evolution of the infant-juvenile mortality rate ratio 2009-2015 Nouna H-DSS.
a: Evolution of 0-59-month mortality rate ratio 2009-2015 in
Nouna H-DSS.
b: Evolution of 0-11-month mortality rate ratio 2009-2015 in
Nouna H-DSS.
c: Evolution of 12-59-month mortality rate ratio 2009-2015 in
Nouna H-DSS.
Two years after the introduction of PCV-13 and Rotavirus vaccines into the national EPI, the impact of these vaccines seems low in Nouna area for all-cause under-five years mortality. In other studies, elsewhere, significant effect has been shown for pneumococcal conjugate vaccine on the reduction of hospitalizations for pneumonia [21,30-35] and invasive pneumococcal diseases (invasive pneumonia, sepsis, pneumococcal meningitis) after introduction of PCV13 vaccine [36,37]. The reduction of diarrhea-related deaths has been observed after introduction of rotavirus vaccine [16,38]. Our data describe a 16% decrease in all-cause under-five years mortality but did not identify a significant PCV-13 and rotavirus vaccine effect on all-cause mortality in children under-five years as seen elsewhere with pneumococcal vaccine and reduction of hospitalizations for pneumonia [32,35,39]. Studies have shown a reduction in all-cause mortality of up to 10% with rotavirus vaccine in children under five years [38,40]. In Malawi where rotavirus vaccine was introduced 2 years after pneumococcal vaccine, a combined 34% vaccine effectiveness was described in the same age group [41].
The use of overall under-five mortality as an endpoint to measure the impact of PCV requires sufficiently similar protocols and data pooling from several countries with high infant and child mortality [18]. Of all possible explanations of the limited impact of both vaccines in our study, the low sample size could be one of those explanation. Other factors could have impacted negatively such as malaria which is a major mortality driver [42] and vaccine coverage. Suarez., et al. [43] in a study conducted in Peru which showed that PCV-13 was not effective in reducing mortality in children under 1 year of age when vaccination coverage was below 85%. Based on the study from Kabore., et al. [44] which found a vaccine coverage of 74% in children under 5 years of age in Burkina Faso in 2015, we hypothesize that vaccine coverage was lower than 85% in our study area.
There are some limitations to our study. The interrupted time series compare the evolution of a health phenomenon before and after an intervention that would alter the natural evolution of this health phenomenon [24,45]. Our study looked at all-cause mortality, which is multifactorial. Some interventions or natural phenomena can influence infant-child mortality (introduction of other vaccines, epidemics, other endemic diseases, other public health interventions).
Our study show that PCV-13 and Rotavirus vaccines were not found to achieve their targeted impact on mortality reduction in children aged 0-59 months. It is likely that the health system was not sufficiently prepared for these introductions and other possibly combined factors. among which an increased malaria mortality, pneumococcus serotype replacement, all-cause mortality, and possible programmatic or vaccine shortage issues such as the Rotavirus vaccine shortage in April-May 2014 might have play a role. However, a reduction trend on mortality was observed in children aged 12-59 months suggestive of possible effectiveness of both vaccines on overall child mortality.
We thank the population of Nouna HDSS, who participated in the longitudinal HDSS data collection, the Noua HDSS field workers, the village key-informant and Nouna village leaders.
No conflict of interest in this work. Neither the author nor any member of his family or relationship is a shareholder or employee of the pharmaceutical companies that manufacture these two vaccines.
Copyright: © 2022 Mamadou Bountogo., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.