Desirée Balconara1*, Salvatore Michele Carnazzo1, Giusi Maria Caltabiano1, Giovanni Cacciaguerra1, Raffaele Falsaperla2, Martino Ruggieri2 and Tiziana Timpanaro2
1 Postgraduate Training Program in Pediatrics, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
2 Department of Clinical and Experimental Medicine, University Hospital “Policlinico-San Marco”, Catania, Italy
*Corresponding Author: Desirée Balconara, Postgraduate Training Program in Pediatrics, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy.
Received: June 08, 2022; Published: August 10, 2022
Citation: Desirée Balconara., et al. “Reliability of Continuous Glucose Monitoring in the Management of Paediatric Dumping Syndrome: A Mini Systematic Review and Our NICU Case Report”. Acta Scientific Paediatrics 5.9 (2022): 05-12.
Background: The role of continuous glucose monitoring (CGM) is widely recognized in the management of the diabetic patient, but is not routinely used to detect pediatric dumping syndrome, especially in infants admitted to NICU.
Aim: The purpose of this study is review systematically the available literature and point out the usefulness of continuous glucose monitoring in pediatric patients for the early interception of dumping syndrome, follow up and orienting therapeutic strategies, proposing the advantage in terms of reliability and tolerability compared to the heel glucose prick with glucometer and suggesting its application in NICU context.
Method: The search is carried on PubMed and Google Scholar database by screening all types of studies, with the keywords “continuous glucose monitoring”, “CGM”, “pediatric dumping syndrome” and these exclusion criteria “ diabetes mellitus” “adult dumping syndrome”. We examinate all publications obtained from the search strategy and assessed the full text of studies potentially relevant for inclusion. Our search identified 9 eligible articles. Moreover, we report our case about a newborn affected by a congenital myasthenic syndrome presenting dumping syndrome.
Results: All the studies cited show the reliability of CGM in detecting glycemic fluctuations, compared to single and intermittent glucose samplings. Glycemic trends helped to adopt the best therapeutic strategy on a case-by-case basis.
Conclusions: Our paper strengthens the evidence on the usefulness of CGM in pediatric dumping syndrome, considering the outcomes reached in all 17 patients studied, in term of safety, reliability, non-invasiveness. Furthermore it suggests the potential future diffusion of this technology that is still little used routinely in NICU departments.
Keywords: Continuous Glucose Monitoring; CGM; Pediatric Dumping Syndrome
Continuous glucose monitoring (CGM) is not routinely used to sue, that lies in place for several days without having to prick the detect pediatric DS; we actually know that it’s primarily applied n diabetes care. CGM is a non invasive device that monitors interstitial glucose level by a sensor inserted in the subcutaneous tissue, that lies in place for several days without having to prick the patient daily. CGM record can be evaluated by many softwares that provide graphical reports, trends and different parameters of glucose homeostasis. The final goal of monitoring glucose is to maintain blood glucose levels within a narrow range, avoiding fluctuations [1]. Infact, glucose variability has been pointed out to play a significant role in intensive care mortality and morbidity such as for neurodevelopmental impairment [2]. Dumping syndrome (DS) is a sequel uncommonly reported in the pediatric age group, mainly following Nissen fundoplication procedure in infants and children with severe gastro-esophageal reflux [3]. Surgical procedures are performed to resolve recurrent episodes of regurgitation, vomiting, poor weight gain, conditions that affected especially newborn and infant with hypotonia, epileptic encephalopathy, severe hypoxic ischemic encephalopathy. Surgical options are used when initial treatment for GERD failed, including frequent small feeding, thickening of the formula and antacid medication. The syndrome emerges after surgery in around 30% of cases [4] because undigested food rapidly entering the small intestine [5] and is clinically characterized by gastrointestinal and vasomotor symptoms [6] such as diarrhea, irritability, diaphoresis, nausea, pallor, vomiting and lethargy, with variable frequency of occurrence and severity. The symptoms are classified as early dumping due to hyperglycemia (within the first hour after a meal) and late dumping (1-3 hours post-prandially), result of reactive hyperinsulinemic hypoglycemia [7,8].
Diagnostic parameters for early dumping syndrome are well established by adult consensus guidelines [9]. However, it is sometimes difficult to diagnose in infants, because their symptoms may be subtle and non-specific, and the glycemic change may be overlooked by a limited frequency of blood sampling. First-line treatment of postprandial hypoglycemia due to late dumping syndrome [10] includes the use of dietary modification with more frequent or continuous feeds and dietary supplementation with fiber, cornstarch, or gelling agents. Second-line therapy is acarbose, an alpha glucosidase inhibitor. Somatostatin analogues are reserved as third-line therapy.
For this systematic review, we searched on PubMed database and Google scholar database using the following keywords “pediatric dumping syndrome” and “continuous glucose monitoring”.
The available records ranged from 2003 to 2022 (last update 01/06/2022). The only filters applied were publication in the English language, human studies, age group “Chlidren” ranging from 0 to 18 years. The exclusions criteria were “diabetic patients” “adult patients”. The review was conducted following the general principles established by Preferred Reporting Items for Systematic Reviews and Meta-Analyses [11] (PRISMA). Because the novelty of this research topic we are aware of the lack of robust evidence [randomized clinical trials (RCTs)] therefore we found predominantly case reports.
The search strategy applied on PubMed database was the following: using the MeSH in brackets (Continuous glucose monitoring pediatric) we identified 615 results; using ((Continuous glucose monitoring) NOT (diabetes)) filtered for Child 0-18, we got 284 results; using (dumping syndrome in children) we obtained 117 results; From each researches, only the articles that responded simultaneously to the dumping syndrome and the use of CGM were screened. In order to achieve this, we used the MeSH ((Continuous glucose monitoring) AND (DUMPING syndrome)) filtered for Child 0-18, so we obtained 12 records, of which 7 results and 5 articles found by citation matching. Four of the five articles were excluded because duplicated or adult patients’ treating. Finally, from PubMed database, 8 articles were eligible.
Searching on Google Scholar database we applied this MeSH (use of CGM in dumping syndrome pediatric CGM ) OR (dumping) NOT adult NOT diabetic, identifying 51 results. We have selected only 1, while all the others have been excluded because they are duplicated of the previous research on PubMed or not inherent.
After analyzing the titles and abstracts of these articles, we included a total of 9 records in this review, that fulfilled all criteria. Five of these are case reports, one case series, one research article, one observational cohort study, one clinical letter. The different phases of articles selection is reported in the diagram 1. In order to ease the access to the information provided by the literature, of each article we summarized in tables the type of article, year of publication, the number of patients included, the treatments. Data are listed in the tables 1. Applying AMSTAR 2, score results low 12. Moreover, we presented our case of a patient with Dumping Syndrome detected by CGM, in NICU-San Marco-Rodolico Hospital in Catania.
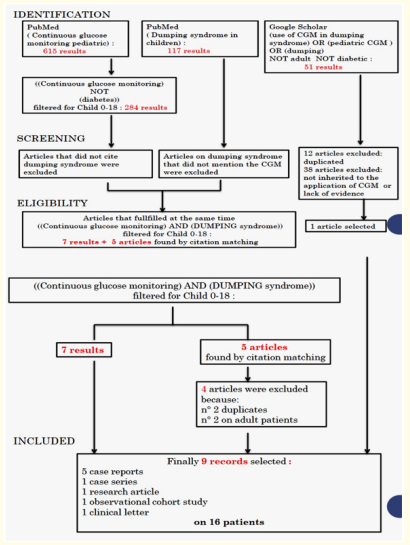
Diagram 1: Study selection process.
All the 9 articles included in this review, showed the advantageous use of CGM as a method to identify dumping syndrome otherwise difficult to diagnose. The total number of enrolled patients with signs and symptoms of dumping syndrome was 16. Of these, 11 patients suffered from severe GERD, 1 affected by congenital central hypoventilation syndrome [13], 4 suffered from tracheoesophageal malformations (3 with congenital esophageal atresia, and 1 with tracheo-esophageal fistula). The outcomes reached are: possibility of identifying glycemic changes in conjunction with the patient’s clinic, early intervention, monitoring of the efficacy of the chosen therapeutic option.
Ueda., et al. [14] published in 2013 a case series on four patients who received Nissen fundoplication: One case had congenital esophageal atresia, and the other three cases had severe central nervous system disorders. They manifested impacting symptoms of GER. Because the failure of Initial treatment, including frequent small feeding, thickening of the formula and antacid medication, the patients received Nissen fundoplication at the age of 3-19 months. Around 4-9 days after the surgery CGM was conducted, detecting remarkable intraday glycemic variability: none of the cases exhibited specific symptoms attributable to early and late DS. CGM also revealed that the lowest level of glucose did not always occur immediately before each feed, the time at when blood glucose is routinely checked. CGM was helpful to confirm the effect of treatment intervention showing a sensible reduction of glucose variability.
Table 1: Articles included in the Review.
In 2016, an italian group [15] reported a case of 4-month-old infant with the diagnosis for esophageal atresia, who underwent traction of esophageal pouches and gastrostomy at 3 months. Since one month after surgery, the baby presented recurrent episodes of diarrea, intense sweating and fine tremors after meals, so CGM was started for a 7-day period: it showed hyperglycemic peaks immediately following meals and then hypoglycemic values after 2 h. Changing feeding schedule, adding starch at evening and fibers in diet, they noted a reduction in glycemic fluctuations with a complete disappearance of symptoms was obtained after a few weeks, visible on CGM profile reported by the authors.
Also Bizzarri., et al. [16] in 2011 published about two children with percutaneous endoscopic gastrostomy; both presented extremely high levels of insulin during hypoglycemia, typical of DS. Using CGM to monitor glucose trend, the authors noted that these episodes disappeared after reposition of the PEG catheter tip for one patient, and the continuous enteral feeding allowed to reach normal glucose balance for the other one.
In a clinical letter of 2019, Morikawa., et al. [17] presented a five-year-old girl with electrical status epilepticus during slowwave sleep (ESES) syndrome complicated by late dumping syndrome who was successfully treated by a ketogenic diet (KD). The KD had the aim of improving not only ESES syndrome but also the late dumping syndrome, expecting that a low-carbohydrate diet would prevent the rapid delivery of carbohydrate into the small intestine and resulting hypoglycemia. To monitor the glucose levels and prevent potential hypoglycemic shock, they used CGM. After reaching a 1:1 ratio on day 25, her glucose trend became stable; at the same time, her epileptic seizures disappeared.
The role of CGM was evident also for monitoring Acarbose therapy in DS. Zung and Zadik [18] described a case of treatment with Acarbose in a 12-months old child subjected to Nissen fundoplication at the age of 6.5 months because recurrent episodes of vomiting and poor weight gain. The authors reported that several therapeutic and dietary manipulations failed to control these symptoms, so they introduced the alpha-glucosidase inhibitor. The novelty of this paper was the way of evaluation of the therapy: they recorded glucose dynamics by a continuous glucose monitor system over 2 to 3 days before and during acarbose treatment, noting that Acarbose attenuated both postprandial glucose hyperglycemia and reactive hypoglycemia [Figure 1].
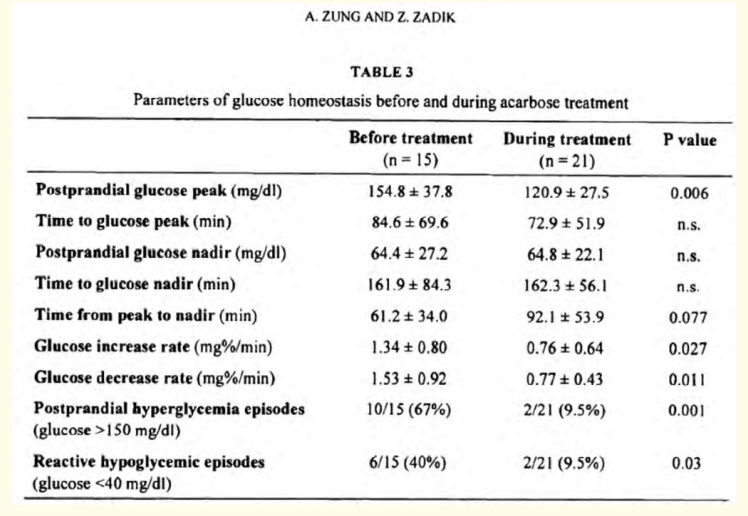
Figure 1: Extracted from "Acarbose Treatment of Infant Dumping Syndrome: Extensive Study of Glucose Dynamics and Longterm Follow-up" Zung and Zadik, 2003.
Vukovic., et al. [19] presented in 2017 the first case of a child with Postprandial Hyperinsulinemic Hypoglycemia following esophageal reconstruction, which responded well to the use of acarbose with dietary modifications. This article is useful to emphasize that although PHH and the DS are now recognized as distinct complications of gastric surgery, both fall within the same “post-bariatric surgery hypoglycemia” spectrum, and the distinction between these two disorders is not always an easy decision to make [20]. However, in the DS symptoms begin shortly after the surgery and improve over time and treatment with dietary changes is usually sufficient. On the contrast, in PHH the symptoms of postprandial hypoglycemia usually begin several months to years after the surgery and pharmacological therapy is usually needed. Also in this last case the benefits of using the CGM in the diagnosis and treatment of hypoglycemia is showed.
In the most recent available case report [21], published in January 2021, the authors described the application of CGM in 3 patients with DS after Nissen fundoplication/tracheoesophageal repair. The glycemic excursions found associated with the absence of hypoglycemic episodes during 11-hours fasting, supported DS diagnosis. The authors also reported [22] that nearly a quarter of children who underwent fundoplication developed postprandial hypoglycemia within one week. Only half of these children exhibited symptoms of DS.
In all of 3 cases, gastric imaging studies had low diagnostic yield. The imaging studies were either normal or displayed results contrary to what would be expected in DS. Thus, the pursuit of multiple imaging studies led to a delay in diagnosis and extension of hospitalization; so CGM revealed essential for diagnosis of DS by revealing the dynamic glucose excursion after feeds.
We present the case of a male newborn affected by a congenital myasthenic syndrome due to compound heterozygosity on the CHRND gene, identified by Clinical Exome Sequencing. Born by emergency caesarean section due to a not very reassuring cardiotocographic trace, from the first minutes of life, he was hypotonic, poorly responsive, with inadequate respiratory drive. Initially ventilated in a nIPPV mode, after in invasive A/C mode for the onset of sialorrhea, absence of the cough and swallow reflex. From 18° day of life until now, invasive ventilation in SIMV mode via tracheostomy was performed. At age of 3 months, he underwent Nissen funduplication and percutaneous endoscopy gastrostomy because poor weight gain, important regurgitation and malarbsorption. He started feeding at the gavage through PEG with anti-colic milk. According to Hammersmith infant neurological examination [23], at 3 months the patient achieves a very low score, because he is unable to maintain the head upright, unable to follow a target, he has poor facial expression, he presents also hypotonia and arthrogryposis of hands and knees. Twenty days later he developed some episodes of profuse sweating associated with irritability after evening meal; Because the recurrence of similar episodes in few days, capillary glucose sampling was established (min 5-max 10 times/day). The episodes began to present daily or multi-daily frequency (about 34) post feeding often, characterized by sweating, pallor, upper limb tremors and irritability. Hyperglycemia (generally 200-300 mg/dL) was found on HGT performed immediately upon observation of the crisis, followed by low blood glucose values after about 3 hours. A relevant observation was that he didn’t develop hypoglycemia during an 11-hour fast. Changes were applied to the nutrition: from gavage feeding we passed to enteral pump feeding with administration every 3 and a half hours. After a week, because the low weight gain, a diet with high-calorie food based on hydrolysed whey proteins was started. For the occurrence of other episodes with similar signs, endocrinological counseling was performed and CGM was placed. The monitoring lasted 14 days, from January 25th to February 14th. The CGM device (Dexcom CLARITY-captur AGP; Dexcom, Inc, San Diego, California) was placed on the lateral side of the thigh. Facilitated tucking and nonnutritive pacifier sucking were used as adjunctive methods to ease pain during the procedure. The Premature Infant Pain Profile (PIPP) score was used to estimate the procedural pain [24] associated with the two skin breaking interventions (CGM insertion and heel glucose prick). We found a score of 4-5 for each heel glucose prick procedure, instead 1-2 points during CGM insertion. During the entire period of CGM the number of records in 24 hours were minimum 288 - max 570. Here we report the most representative daily CGM profiles of the patient. First day of CGM application is reported in figure 2; A similar glycemic variability was found in the following days, confirming the suspected DS, statistically during the first week: 87% of records were in optimal range (70-180 mg/dL) 5% of records were below the optimal range (2% of severe hypoglicemia) 8% of records were above the optimal range. In consideration of the observed glycemic profile, it was therefore decided to start a continuous enteral feeding since February 1th. Following the transition from discontinuous to continuous enteral feeding, there was a rapid and visible normalization of the glycemic profile [Figure 3], progressive weight gain and improvement of skin and muscle trophism. The 95-100 % of records were in optimal range.
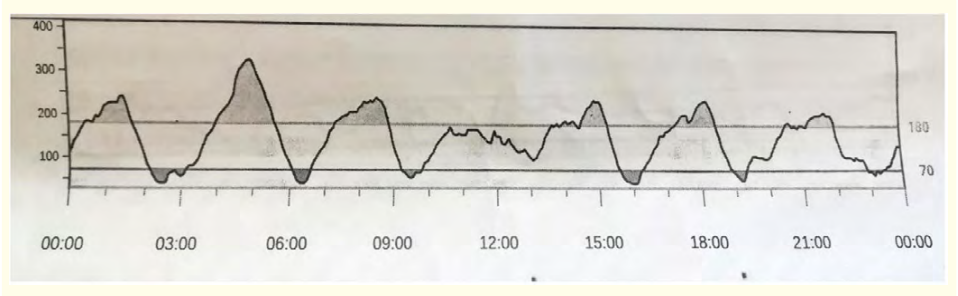
Figure 2: First day of CGM record. Feedings number: 7 (at h 3:00, 6:30, 9:00, 13:00, 16:30, 20:00, 23:30) Glicemic values expressed in mg/dL on the vertical axis. Time in hours on the Horizontal axis.
Because the articles reported are predominantly case reports and case series, the number of patients in each article is remarkably low. This agrees with the novelty of the subject matter, considering that in the large literary series of pediatric dumping syndrome only in a few cases continuous glucose monitoring has been applied. The interest in the potential of CGM in fields other than diabetic is quite recent, just think that the oldest article dates back to 2003. Discussing the results extrapolated from the selected articles we could assert the usefulness of the application of CGM in pediatric patients with presumable dumping syndrome, highlighting the objective difficult to intercept its symptoms in another way and the importance of an early diagnosis for timely management and better outcome. The last decade emerged an expanding interest on glucose homeostasis disorders in patients needing intensive care, because it has proven to play a significant role in infant morbidity and mortality. A more accurate control of glucose curve is increasingly possible thanks to the application of the CGM devices in clinical practice. The demand for more accurate clinical follow up has led to the introduction of several glucose variability parameters. However, there has been few evidence of which variability parameters could best characterize the severity of illness. Most accepted parameters [25] are: Mean of Daily Differences (MODD), Mean Amplitude of Glycemic Excursions (MAGE) and Continuous Overall Net Glycemic Action (CONGA). Recently, softwares have been developed and found useful for the calculation of these parameters. Despite great advances in the technology, current devices are not designed for use in babies and there are technical challenges with using devices in the newborn which include: insertion methods, accuracy, clinical interpretation [26]. We could summarize the current limitations [27] as follows:
The wealth of data available from CGM makes it attractive for use in NICU where many physiological variables are measured continuously. The benefits in NICU may be greater as blood glucose sampling is much less frequent, in keeping with the aims of minimal handling and limiting blood loss as well as the importance of the potential impact of reducing dysglycemia on the vulnerable developing brain; it also allows to reduce the trauma of multiple peripheral blood samples using a glucometer and consequently reduce the algic impact. Given the dynamic nature of glycemic changes during dumping syndrome, it is not surprising that many abnormal blood glucose events may be overlooked by intermittent blood sampling.
In conclusion, our report underscores the usefulness of CGM in children with suspected DS. CGM resulted effective for both the diagnosis and the management of DS. Clinicians should maintain a high index of suspicion for dumping syndrome when evaluating children with a history of gastric surgeries, even if the child exhibits no or few clinical symptoms.
Although current technology is not specifically designed for use in NICU and there remain clinical challenges in interpretation of the data and implications for everyday clinical practice, the rapid technological developments in sensor technology hold the potential for us to optimize the management of glucose levels in this vulnerable population. Therefore the CGM would appear to be safe, reliable, tolerable especially when compared to blood glucose measurement. Thus, our case might also serve as a clinical reference for future studies.
Copyright: © 2022 Desirée Balconara., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.