Kristine M Prayon and Anabella S Oncog*
Department of Pediatric Medicine, Government Celestino Gallares Memorial Hospital, Philippines
*Corresponding Author: Anabella S Oncog, Department of Pediatric Medicine, Government Celestino Gallares Memorial Hospital, Philippines.
Received: May 30, 2022; Published: June 13, 2022
Citation: Kristine M Prayon and Anabella S Oncog. “Absolute Neutrophil Counts and Neutrophil to Lymphocyte Ratio as Early Predictive markers of Dengue Severity among Children admitted in Governor Celestino Gallares Memorial Hospital: A 5-Year Retrospective Study”. Acta Scientific Paediatrics 5.7 (2022): 37-45.
Background: Dengue fever is known to have an unpredictable course and outcome. Because of this uncertainty, diagnostic tools for early detection of severe dengue should be developed. Studies on the correlation of degrees of neutropenia and Neutrophil-Lymphocyte Ratio (NLR) with dengue severity on the pediatric population are scarce, and its clinical significance is still uncertain.
Objective: To determine the correlation of Absolute Neutrophil Counts (ANC) and Neutrophil-Lymphocyte Ratio (NLR) during the acute phase of dengue infection with the development of severe dengue among children admitted at Governor Celestino Gallares Memorial Hospital (GCGMH).
Methodology: This is a retrospective descriptive correlational study. The charts of children aged 0-18 years with laboratory-confirmed dengue who were admitted to GCMGH from January 1, 2016 to December 31, 2020 were reviewed. Data collection was done using simple random sampling. Data were analyzed using frequency, percentage, median and standard deviation. Chi-square test was used to determine the association of ANC and NLR with dengue severity.
Results: Out of 912 eligible patients, 584 subjects were enrolled. Abnormal ANC was noted in 41.4% of subjects where, 21.6% had mild neutropenia, 17.8% had moderate neutropenia and 2.1% had severe neutropenia. Almost 44% of subjects had high NLR. There was no significant association noted between ANC and dengue severity. However, a trend towards increasing risk for severe dengue was noted with increasing severity of neutropenia. No significant association was also noted between NLR and dengue severity.
Conclusions: There is no significant correlation between neutropenia during the febrile phase of dengue infection with the development of severe dengue in pediatric patients. However, a trend towards increasing risk for developing severe dengue as the severity of neutropenia increases was noted. High NLR during the febrile stage of dengue, likewise, is not significantly associated with progression to severe disease.
Keywords: Absolute Neutrophil; Counts; Lymphocyte Ratio; Dengue Severity; Children.
In recent years, dengue has become one of the most significant and fast emerging tropical viral diseases with incidence growing dramatically especially in the Philippines. Its unpredictable clinical evolution and outcome has been one of the major challenges encountered by healthcare providers. Most patients recover from a non-severe clinical course while some progress to severe disease. With this as a background, it is therefore prudent to develop diagnostic tools for early detection of impending severe dengue disease.
Several studies have used different clinical and laboratory parameters in an attempt to predict the development of severe dengue infection during the acute febrile phase of the disease. Majority of these have used laboratory parameters as early predictive tools for dengue severity. Dengue fever is known to be a non-inflammatory type of disease. Therefore, inflammatory biomarkers such as Neutrophil-Lymphocyte Ratio (NLR) is not expected to rise in dengue fever. Also, one of the features of dengue virus is its ability to suppress bone marrow production of leukocytes specifically neutrophils leading to absolute neutropenia. Recent researches have utilized absolute neutrophil count (ANC) [1-4] as an early indicator of severe dengue. However, these studies warrant further validation in other regions and age groups.
To address this particular research gap, this study will utilize ANC and NLR as early predictive markers of dengue severity in an attempt to establish a correlation between the degree of neutropenia and high NLR to the development of severe dengue infection in the pediatric population.
This research paper is believed to benefit the following stakeholders:
This study supports the institution’s vision and mission to become a premier research facility in region 7.
This study will be potentially useful for clinicians in resourcelimited hospitals and in dengue endemic regions in order to identify patients who are at risk for developing severe dengue at early stages of illness, to lessen or avert the development of severe dengue, and to administer needed care earlier, thereby, improving outcomes of dengue fever in the country.
The Boholano children will be assured of the best management of dengue fever that the institution can provide.
To determine the correlation of Absolute Neutrophil Counts (ANC) and Neutrophil-Lymphocyte Ratio (NLR) during the acute phase of dengue infection with the development of severe dengue among children admitted at Governor Celestino Gallares Memorial Hospital from January 1, 2016, to December 31, 2020.
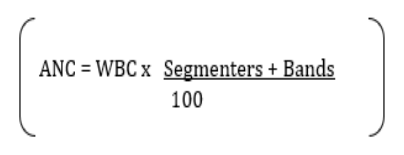
Dengue fever is a mosquito-borne disease caused by at least four (4) distinct antigenic types of dengue virus (dengue 1, 2, 3, and 4), members of the family Flaviviridae [5]. The World Health Organization (WHO) now considers dengue as a major global public health challenge in the tropics and subtropic nations with an estimated 50-100 million infections occurring annually in over 100 endemic countries [8].
Globally, there is an estimated 390 million dengue infections per year; 96 million of which manifest clinically [9]. Dengue is now endemic in more than 100 countries. The most seriously affected are regions in America, South-East Asia, and the Western Pacific wherein, cases of dengue exceeded 1.2 million in 2008 and over 3.34 million in 2016. Of the 375,000 suspected cases of dengue in 2016 in the Western Pacific Region, 176,411 were cases reported from the Philippines [10]. In 2019, a total of 146, 062 cases with 622 deaths were recorded in the Philippines since January to July 20 which led to the declaration of National Dengue Epidemic from the Department of Health (DOH) [11]. In Region 7, the DOH recorded a 118 percent increase in dengue cases this year compared to the same period last year [12].
Dengue has a broad spectrum of clinical presentations often with unpredictable clinical evolution and outcome, ranging from probable dengue, dengue fever with and without warning signs and severe dengue [13]. While most patients recover following a self-limiting non-severe clinical course, a small proportion progress to severe disease.
Because the signs and symptoms of dengue fever are non-specific, laboratory confirmation is important. A confirmation of dengue case is done by isolation of the virus, serological tests (Dengue IgG, IgM), viral antigen (NS1), or by molecular methods [14].
Severe dengue has become a leading cause of hospitalization and death among children and adults in Asian and Latin American countries recently [15]. Complications of dengue primarily due to plasma leakage develop around the time of defervescence. During the acute febrile phase, it is difficult to predict or to distinguish who among those diagnosed dengue patients will succumb to severe dengue during the critical phase. Because of this uncertainty, the development of diagnostic tools for early detection of impending severe dengue is warranted and should be prioritized to reduce the morbidity and mortality rates of dengue infection [16]. To date, there are no prognostic tools available to distinguish severe dengue from non-severe dengue at early stages of the disease.
Several studies have been conducted using different clinical and laboratory parameters as early predictive markers of severe dengue. Those who utilized the presence of specific warning signs as early predictors of dengue infection severity found support that the presence of vomiting [17-19]. abdominal pain [17,20] and bleeding [20,22] were associated with the development of severe dengue. Others have linked demographic features such as female sex [17,22] and younger age [22-24] to higher severe dengue incidence. Majority have considered laboratory parameters such as LDH and albumin [25], levels of CRP [1,25], elevated AST [18,23,24], high NS1 levels [18,26], WBC and WBC differentials [23,27], hematocrit levels [23,27] and cfDNA [28] as early predictors of severe dengue. However, all these studies need further validation in other regions and age groups.
The clinical course of a viral infection can be adversely affected by bacterial co-infection, as concluded by several studies [29-31]. One study in 2005 has identified 5 patients with severe Staphylococcus Aureus co-infection arising from the same worksite amongst 4,000 dengue cases admitted in a hospital in Singapore [32]. There are also about 20 other isolated and sporadic cases of bacterial coinfection with dengue reported in the literature in which majority involved co-infection with gram-negative Enterobactericeae species [33-35].
Neutrophils are the first leukocytes to migrate to sites of inflammation and infection where they recognize and phagocytose invading microorganisms. Leukopenia and absolute neutropenia have been described as a feature of dengue infection due to bone marrow suppression by dengue virus [36]. In general, individuals with severe neutropenia are at higher risk of secondary bacterial infections, however, it is still uncertain whether dengue patients with severe neutropenia are more prone to secondary bacterial infections. Studies on the correlation of neutropenia with progression to severe dengue disease are scarce. One study showed that any change in the values of total leukocyte count points towards the progression of dengue towards severity [2]. Similar studies done by David Chadwick., et al. [3] in 2006 and Tzong-Shiann Ho., et al. [4] in 2013 found that neutropenia has positive predictive values for dengue fever and its severity [3,4].
Another useful biomarker of inflammation is the Neutrophil to Lymphocyte Ratio (NLR). It has proven its prognostic value in cardiovascular diseases, infections, inflammatory diseases and in several types of cancers. Dragan Djordjevic., et al. demonstrated in their study a clear relationship between higher NLR levels and lethal outcome in critically ill adult patients with peritonitis and pancreatitis [37]. A similar study done in the pediatric population reported that a rise in NLR helps in predicting the mortality in the pediatric intensive care unit [38]. Another study assessed the ability of NLR to predict sepsis in children and concluded that high NLR values should alert clinicians to the possibility of sepsis and to initiate or change antibiotic treatment [7].
To date, only one study has been conducted which attempted to establish a correlation of neutrophil counts as an early indicator of severe dengue in children [1]. This study involved all pediatric children admitted to a tertiary care center with laboratory- confirmed dengue fever from June 2014 to June 2016. The authors of this study concluded that neutropenia (< 1,500/cmm) can serve as an early predictive marker for severe dengue especially in a resource-limited setting. However, a major limitation of this study is that it did not include many severe dengue cases since there were no enrollees who developed severe dengue.
In this regard, this study will attempt to answer this particular research gap. Moreover, there is limited published data in the Philippines at present that utilize different laboratory parameters as early predictive tools for the development of severe dengue.
This is a retrospective descriptive correlational study.
Governor Celestino Gallares Memorial hospital is an apex hospital in the province of Bohol, hence, it receives referrals from other hospitals in the different municipalities. Data from the institution’s dengue surveillance showed that pediatric dengue patients who were admitted from January to December 2018 came from 42 out of 47 municipalities in the province.
Fluid management plays a crucial role in the development of complications leading to severe dengue, however, different physicians have different approaches in terms of fluid management in dengue which may be a major confounding factor in this study. To ensure uniformity in fluid management, this study will only include patients who will be admitted to Governor Celestino Gallares Memorial Hospital pediatrics ward with laboratory-confirmed dengue fever.
The sample size was computed using the Open Epi version 3.In this study, the sample size was computed for each year (20162020) with a 95% level of confidence. The computed minimum sample size is 125, 63, 131, 187 and 78 subjects for the year 2016, 2017, 2018, 2019 and 2020, respectively, giving a total of 584 subjects.
The subjects were chosen using simple random sampling technique.
All patients who were admitted to the pediatrics ward of Governor Celestino Gallares Memorial Hospital, aged 0 to 18 years, with laboratory-confirmed dengue fever within the first 72 hours of illness onset were included in the study.
Excluded from the study were patients with
This study included eligible patients admitted from January 1, 2016 to December 31, 2020. Data gathering commenced on June 1, 2021 and ended on September 30, 2021.
All data obtained were properly noted and tabulated. Descriptive statistics was used in describing the data gathered. Categorical variable was described using frequency and percentage while continuous variable was summarized using median and standard deviation. On the other hand, Chi-square test was used to determine the association of ANC and NLR with dengue severity. The point and 95% confidence interval of the relative risk was computed to determine the magnitude of the association. All data were analyzed using IBM Statistical Package for Social Sciences (SPSS) version 20.0.
There were 2,762 pediatric dengue cases admitted to the pediatric wards of Governor Celestino Gallares Memorial Hospital from January 1, 2016 to December 31, 2020. Nine hundred twelve patients aged 0 to 18 years, with laboratory-confirmed dengue fever within the first 72 hours of illness onset were eligible based on the inclusion and exclusion criteria, of which 584 patients were enrolled in the study. Their age ranged from 3 months to 18 years with a mean of 9.38 years (sd = 4.42 years). There were more males than females with 55.7% and 44.3%, respectively.
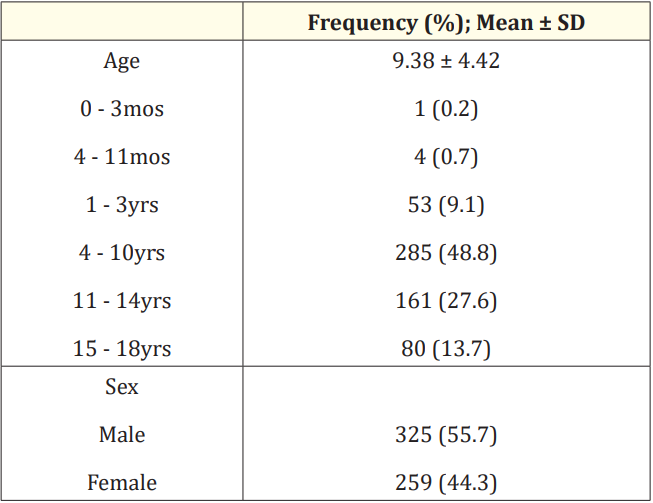
Table 1: Demographics of the study cohort (N = 584).
Table 2 depicts the distribution of subjects according to laboratory parameters. Abnormal ANC was noted in 242 (41.4%) of subjects where, 126 (21.6%) had mild neutropenia, 104 (17.8%) had moderate neutropenia and 12 (2.1%) had severe neutropenia.Almost 44% of subjects had high NLR.
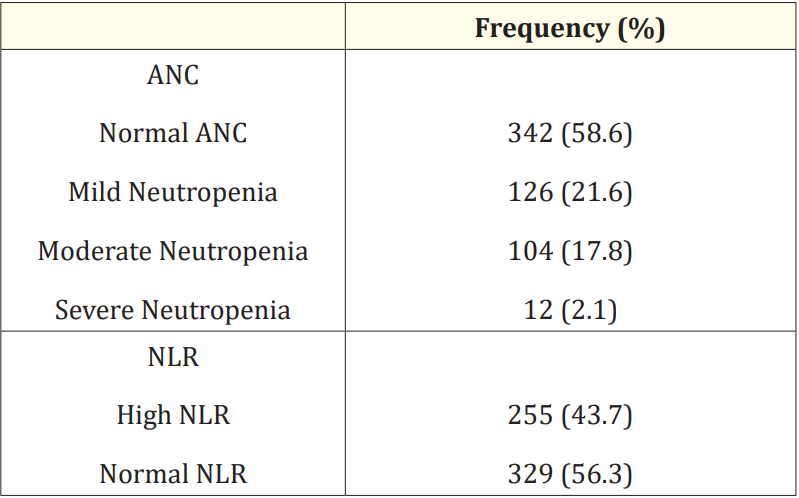
Table 2: Laboratory parameters on admission of the study cohort (N = 584).
Table 3 shows the association of ANC and NLR with dengue severity. Of the 584 patients with laboratory-confirmed dengue fever, 33 (5.7%) were found to have severe dengue and 551 (94.3%) non-severe dengue. Based on the results of the study, there was no significant association noted between ANC and dengue severity as shown by all p values > 0.05. However, a trend towards increasing risk for severe dengue was noted with increasing severity of neutropenia. This was shown by the increasing relative risk for mild, moderate and severe neutropenia of 0.90, 1.46 and 1.58, respectively.
The results also showed no significant association noted between NLR and dengue severity (p = 0.88).
Dengue fever remains to be a significant threat to public health globally. In the Philippines, surveillance data showed 794,255 annual dengue episodes from 2010 to 2014 which is 10 times higher than cases of rabies, twice the cases of intestinal flukes and about 10% of the burden of tuberculosis [39]. Dengue is still considered as one of the major causes of morbidity and mortality especially among Filipino children. On the dengue situation update released by WHO, Philippines has recorded a total of 32,555 dengue cases with 119 deaths from January 1 to July 3, 2021[40].
Majority of the patients with dengue were between 4 to 10 years old which is similar to a study done in a tertiary hospital in Eastern India in 2019 where most of the dengue-afflicted patients involved also belong to the 4-10 years old age group [41]. This data is also similar to the findings of other Philippine-based studies on dengue fever by Lim., et al. [42], Bravo., et al. [43], Capeding., et al. [44], and Oncog and Pondoc [45]. This could possibly be related to outdoor activities of these children and therefore more likely to experience mosquito bites.
From a sample of 584 pediatric dengue patients, it was found that the incidence distribution is higher in males which accounted for 55.7% of the patients involved in the study. This is in congruence with the report from WHO from January 1 to March 29, 2008 which showed that majority of dengue cases were male [46]. Similar proportions were also found in several studies done on children with dengue fever in India [41,47-50] and Indonesia [51] where dengue is also endemic. Data on sex distribution of pediatric dengue patients in the Philippines, on the other hand, are varying. Studies done by Lim., et al. [42] and Capeding., et al. [44] showed an equal sex distribution among children with dengue fever. In a prospective cohort study of 42 dengue cases admitted in Cardinal Santos Medical Center, San Juan (NCR) from November 2006 to August 2007, more dengue cases were recorded in females [52]. Similarly, a study done in Tagbilaran City by Oncog and Pondoc [45] showed that dengue infection was more frequent in females than in males which, however, contradict the results of this study which was done in the same locale. This discordance may be due to lower age limit (0-14 years) set by the authors for the subjects involved as compared to the higher age limit used in this study (0-18 years). The number of institutions included in the studies may also be a contributing factor to the opposing results of sex distribution of pediatric dengue patients in Tagbilaran City.
In our study, most cases had non-severe dengue (94.35%) and only 5.7% developed severe dengue during their hospitalization. This result is comparable to the studies done in India by Purkait and Basu [41], Mishar., et al. [47] and Pothapregada., et al. [48] where only 12.73%, 13.4% and 39.5% of the cases included had severe dengue, respectively. Despite the low incidence of severe dengue recorded, the mortality rate of dengue remains to be high specifically in children where 42% of deaths recorded from dengue in the first 7 months of 2019 in the Philippines were children between 5 to 9 years old [53]. These data are in contrary to the results from studies in Mexico by Alvarado-Castro., et al. [54] and in the Philippines by Capeding., et al. [44] where majority of the cases included had severe dengue. Regardless of these contradicting results, more efficient strategies for the prevention and early detection of dengue and its progression to severe disease should be developed.
It is known that bone marrow suppression is one of the hallmarks of dengue fever which affects all cell populations in the bone marrow [55]. Though less frequently, neutropenia in dengue infection has been reported and studies on correlation of neutropenia with dengue severity are scarce, both in the pediatric and adult populations.
This study has found that neutropenia was present in 41.4% of the subjects involved. However, neutropenia in our study was not significantly associated with the development of severe dengue. This supports the findings from several studies done on correlation of ANC and dengue severity. In an observational study done by Khandelwal R. and Khandelwal L.M. on 50 patients aged 1 to 14 years in Bangalore, it was reported that most of the patients in febrile phase of dengue had neutropenia which was also not related to secondary bacterial infections and to progression to severe disease [2]. It was also concluded in a study of 1,921 laboratoryconfirmed dengue patients in Singapore that severe neutropenia was not predictive of more severe disease and not associated with secondary bacterial infections, prolonged hospital stay, prolonged fever, or fatal outcome [56]. However, this study involved adult dengue patients. On the other hand, data from a study which used ANC levels as predictive marker of severe dengue suggested that neutropenia (ANC: < 1,500) may serve as an early predictor of severe dengue [1]. Chadwick D., et al. [3] and Ho TS., et al. [4] also found that neutropenia has high predictive value for the development of severe dengue. However, majority of the subjects included in these 2 studies were adults (> 18 years old), hence, the results may not be analogous in the pediatric population.
Despite the absence of significant association of ANC and dengue severity in our study, it is important to note that the results revealed a trend towards increasing risk for developing severe dengue as the severity of neutropenia increases.
There is still a controversy on the ideal cut-off of NLR due to inadequate number of studies. In this research, we used the cut-off value of 1.97 which was the optimal cut-off value with a 75.6% sensitivity and 38.4% specificity identified in a study where NLR was used as one of the useful biomarkers to predict sepsis in children [7]. It was concluded in the study of Rini., et al. in 2020 that high NLR values were significantly correlated with bacterial infections to that in viral infections such as dengue [57].
Based on this research, there is no significant association between high NLR during the febrile phase of dengue and progression to severe disease. This is in concordance to results shown in few studies where NLR was used as one of the prognosticators of severe dengue. In a retrospective study in Indonesia using 52 patients with dengue, it was found that lower NLR levels, not high, were significantly associated with the increase in degree of dengue severity [58]. Similarly, Karla., et al. s study in 2015 concluded that there was no significant correlation between NLR levels and dengue severity grade [59]. Yuditya and Sudirgo’s research in January 2019 on the relation of NLR and dengue infection grade severity among 76 patients infected with dengue virus, likewise, showed that the lower the NLR, the dengue infection grade will be more severe [60]. These studies, however, were done on adult patients.
Just recently, one retrospective study has been conducted in the Philippines where the authors used NLR as one of the prediction models of severe dengue in pediatric patients admitted at Philippine Children’s Medical Center (PCMC) from 2016 to 2019 which showed a contrast result to this research. Results of this particular study showed that 22% of 287 dengue patients enrolled progressed to severe dengue and along with high WBC count, high NLR on Day 4 of illness significantly predicted progression to severe dengue [61]. To date, this is the only published study where the predictive value of NLR for severe dengue was utilized in children with dengue in the Philippines. We postulate that the discordance of the result of this study with the findings of our research may be due to the different cut-off values used by the investigators and the day of illness the CBC results were considered. A lower cut-off value of 0.93 was used by Barcelona., et al. in their study in PCMC while a higher cut-off value of 1.97 for NLR was used by the authors of this study. This might have resulted to higher number of patients with high NLR values in their study as compared to the number of patients with high NLR included in our research. CBC results considered in our study were those taken on Day 1 to 3 of illness (febrile phase) while in the PCMC study, CBC indices which showed high NLR values that were significantly associated with progression to severe dengue was noted on Day 4 of illness. Moreover, the number of subjects included in our study (N = 584) is almost 50% higher than those enrolled in the PCMC study (N = 287).
In this study, we conclude that there is no significant correlation between neutropenia during the febrile phase of dengue infection with the development of severe dengue among children admitted in Governor Celestino Gallares Memorial Hospital. However, a trend towards increasing risk for developing severe dengue as the severity of neutropenia increases was noted. High NLR during the febrile stage of dengue (first 72 hours), likewise, is not significantly associated with progression to severe disease.
One of the primary limitations to the generalization of the results of this study is the lack of a standard cut-off value for NLR in children up to the present. Further studies are warranted to be able to come up with a standard normal value of NLR in healthy children.
Due to limited published studies on ANC and NLR as early predictive markers of dengue severity and some discordance of the results of this study with published ones, it is recommended that further studies be made in different regions involving larger samples which may determine the true predictive values of ANC and NLR in dengue severity.
Further, with extensive literature search, this study is the only study done on Filipino children which utilized ANC and the second to use NLR in children with dengue as early predictive tools for severe dengue. More studies should be conducted in the Philippines in order to determine their practicability in predicting severe dengue among Filipino children during the febrile phase of illness which can lead to better management and lesser mortalities among children infected with dengue.
Lastly, the increasing risk for developing severe dengue with increasing severity of neutropenia which was noted in this study should alert pediatricians to consider ANC levels in the management of children with dengue fever.
Copyright: © 2022 Kristine M Prayon and Anabella S Oncog. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.