Fares Florence*
Department of Pediatrics, Saint-Joseph University/CHU-Hotel Dieu de France de Beyrouth , Lebanon
*Corresponding Author: Fares Florence, Department of Pediatrics, Saint-Joseph University/CHU-Hotel Dieu de France de Beyrouth, Lebanon.
Received: March 29, 2022; Published: April 28, 2022
Citation: Fares Florence. “Organochlorine Pesticides in the Breast Milk of Lebanese Women: Magnitude of the Environmental Burden 25 Years After Banning their use”. Acta Scientific Paediatrics 5.5 (2022): 58-64.
Breast milk contamination with organochlorine pesticides reflects long periods of inadequately and sometimes uncontrolled pollution of the environment. Among other fat-soluble substances, they accumulate in fatty tissues, get biomagnified through the food chain and are subsequently transferred from lactating mothers to their offsprings. Their toxic effects are dose related. The goals of this study were to determine the levels of organochlorine pesticides in the milk of lactating mothers in the region of Beirut years after banning of their use. 60 samples of breast milk were collected, and mothers were asked about their demographic features and behavioral and dietary habits. We used the CPG-MS method for organochlorine levels measurements. 55% of samples were tested positive for at least one organochlorine pesticide and 16.7% of these levels exceeded Maximal Residue Limits. DDE, a metabolite of DDT is the most encountered contaminant (35% of samples). Lindane levels are the most toxic. No correlation was found between the levels of organochlorine pesticides and the demographic and behavioral features of the population. Fish consumption was correlated with p-p’DDE levels (p = 0.036), poultry consumption was correlated with p-p’DDD (p < 0.01), and total DDT levels (p < 0.01) and milk consumption was correlated with HCB levels (p = 0.023). No correlation was found between organochlorine pesticides levels and maternal consumption of fruits and vegetables.
Keywords: Organochlorine Pesticides; DDT; Lindane; Breast Milk; Bio-Indicator; Toxicity; Pollution; Environment.
Many pollutants and chemicals currently released into the environment constitute a real hazard for public and infantile health. Others, more worrisome, continue to be harmful despite the prohibition of their use. These inherited substances are known by the acronym of POPs (Persistent Organic Pollutants). They are divided into three main categories: organochlorine pesticides, dioxins and furans and Polychlorinated biphenyls (PCBs). They are lipophilic synthetic organic compounds that persist in the environment for many years, resist biodegradation and accumulate in fatty tissues. Almost all living organisms have measurable levels of POPs in their body [1]. These substances are found in food, accumulate in breast milk and are transmitted from mothers to their offspring during breastfeeding. Many studies determining the level of POPs in breast milk have been conducted [2-5]. The use of these products was banned in the late 1970s in most industrialized countries and in the late 1980s in most developing countries [6]. The Stockholm Convention on Persistent Organic Pollutants [7] ratified by 151 countries including Lebanon in May 2001, aimed at the global elimination of the twelve most dreaded POPs. Nine of these twelve products are organochlorine pesticides used in agriculture and in pest control. In Lebanon their use is limited to agriculture. They have endocrine [8], immunomodulatory [9], teratogenic and oncogenic properties [10] and have been associated with psychomotor developmental delays [11,12], cryptorchidism [13] and spontaneous abortion [14]. In populations where exposure exceeds recommended doses, neurodevelopmental disorders [15-17], endocrine disorders [18-21], immunological toxicity [22,23], and an increased incidence of certain cancers [24,25] have been reported. In this study we measured the impregnation of Lebanese mothers’ milk with organochlorine pesticides. The last similar study carried out in Lebanon dates back to 1999 (Dagher., et al). Our objectives were 1) to detect possible recent contaminations, 2) to compare the levels obtained with the maximum limits tolerated in food, 3) to compare them to the levels previously reported in Lebanon and 4) to determine the eating habits and factors in lifestyle which influence them the most.
The study population is made up of sixty Lebanese women aged 17 to 40 (mean age ± SD 29.4 ± 5.9). They were recruited from the clinics of different pediatricians in the Beirut region in 2010. The recruitment was realized on a voluntary basis and was approved by the hospital’s ethics committee. After signing an informed consent, the subjects completed a questionnaire concerning their demographic features (address, age, weight, height, parity, duration of breastfeeding, smoking, current medications) and their eating habits (nature, frequency and quantity). Samples of 10 ml of human milk were obtained by manual expression at different stages of lactation. They were collected in decontaminated glass tubes and stored at 0°C until the dosage was performed. We used the CPG-MS method for the dosage. The analysis was performed by gas chromatography-tandem mass spectrometry. The statistical analysis was realized by SPSS (version 16.0). Results are reported as mean and standard deviation (m ± SD). Pearson correlation coefficient was used for linear correlations between variables. Only values of P < 0.05 were considered statistically significant.
The demographic characteristics of the population are presented in table 1. 23% of the women recruited for the study were primiparous. 77% have two to six children and have already breastfed. The average duration of breastfeeding before sampling is 147.2 days ± 114.9. 15% had a premature delivery. 35% are smokers. The average weekly consumption of milk, poultry, meat and fish is presented in table 2.
The average and maximum concentrations of the pesticides tested are summarized in Table 3. Values are expressed in μg/L. figures 1 and 2 show the distribution of organochlorine pesticide residues in the study samples. The detection threshold is 0.1μg/ mL. The values exceeding the maximum residue limits (MRL) are reported in bold on figure 2. DDT metabolites are the most encountered organochlorine pesticide residues in the study samples (25 samples or 41.7% are positive for DDE and/or DDD). p-p’ DDE is present in 35% of the samples. The average DDE level is 2.92μg/L ± 8.08. In the samples tested positive, the levels vary from 0.1 to 42µg/L. They exceed the MRL for DDE (0.036 mg/kg) [26] in two samples. p-p’ DDD is present in 13% of the samples. The average rate of DDD is 1.10μg/L ± 4.86. The levels vary from 0.15 to 32.2µg/L and do not exceed the maximum residue limit. p-p’DDT and o-p’ DDT were found in only one sample. The value of total DDT in this sample is 24.57µg/L and exceeds the MRL for DDT (0.02 mg/Kg). Eight samples (13%) tested positive for Lindane. The average rate of Lindane is 5.90 μg/L ± 1.94. Seven values exceed the MRL for Lindane (0.01mg/kg) [27]. Five samples tested positive for HCB. Only one value exceeded the MRL for HCB (0.01 mg/kg) [28]. Two samples tested positive for heptachlor epoxide (HE) and only one for Aldrin. The values reported do not exceed the MRLs (1mg/ kg, 0.004 mg/kg and 0.006 mg/kg respectively) [29-31]. In our study, no relationship was established between the levels of pesticides measured and age, parity, BMI, duration of breastfeeding, smoking, place of residence and the use of detergent for washing fruits and vegetables. A bivariate correlation analysis was realized between pesticide levels in breast milk and feeding habits. It showed positive correlations between poultry consumption and the levels of p-p’DDD (r = 0.414; p = 0.001) and Total DDT (r = 0.530; p = 0.001), between fish consumption and p-p’DDE levels (r = 0.271; p = 0.036) and between milk consumption and HCB levels (r = 0.294; p = 0.023). No significant correlation has been demonstrated between the consumption of fruits and vegetables and the level of pesticides in breast milk.
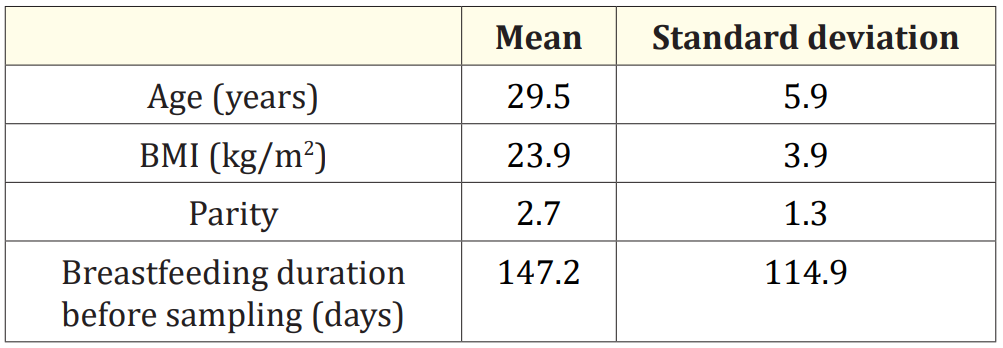
Table 1: Demographic characteristics of the study population.
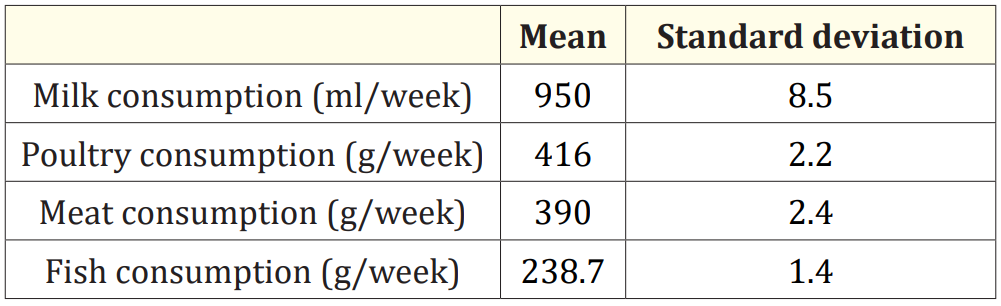
Table 2: Weekly consumption of animal products.
The use of organochlorine pesticides became widespread after the Second World War. These products initially had the advantage of limited acute toxicity. Concerns about their chronic toxicity and persistence led many countries to ban their use in the 1970s. Since its founding in 1949, the World Health Organization (WHO) has considered food safety as the main component of its global health protection program. In Lebanon, the use of many pesticides was banned in the late 1980s (1985 for DDT) and the list of POPs deemed dangerous is continually updated by the Ministry of Health [6].
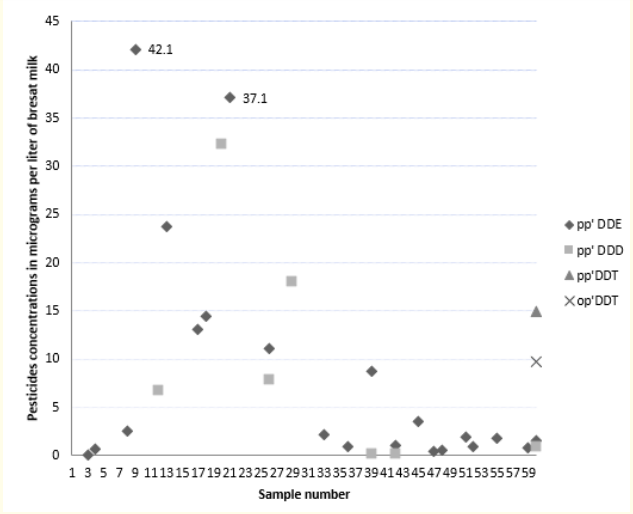
Figure 1: Levels of DDT and its metabolites in the study samples.
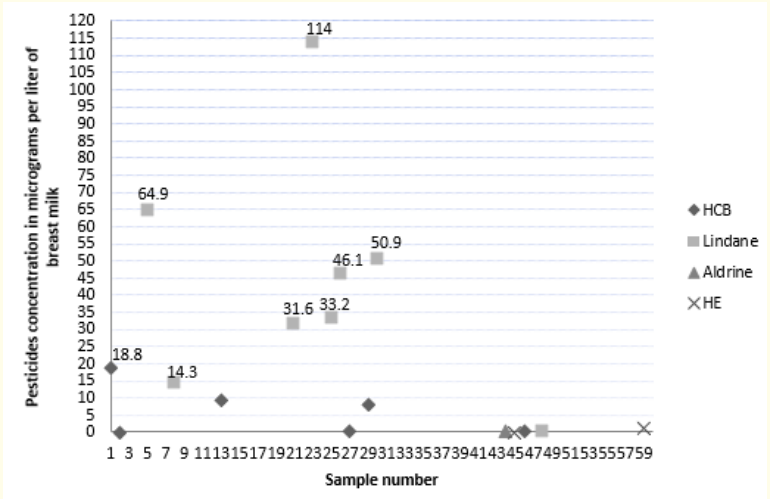
Figure 2: Levels of HCB, Lindane, Aldrin and Heptachlore Epoxyde in the study sample.
Our results suggest that breast milk contamination of Lebanese women by organochlorine pesticides is not negligible. 55% of the samples studied tested positive for at least one and 13.3% for at least two organochlorine pesticides. In accordance with data from the literature [1], DDT and its metabolites are the main encountered pesticides (Figure 3). DDE rates are lower than those previously reported in Lebanon in 1999 [4]. Even if the concentrations vary greatly from one region of the world to another, since the application of the measures prohibiting their use, many studies have also reported a trend towards a decline of POPs levels in breast milk [2,32,33]. Our total DDT levels are also lower than the rates reported in Brazil in 2000 [34] and 2002 [5], Mexico in 2000 [4] and China in 2004 [35]. This can be explained by the fact that the use of DDT in Lebanon was limited to agriculture: in certain regions of the world such as Africa, Mexico and Brazil, it is still used in parasite control and the fight against vectors of malaria.
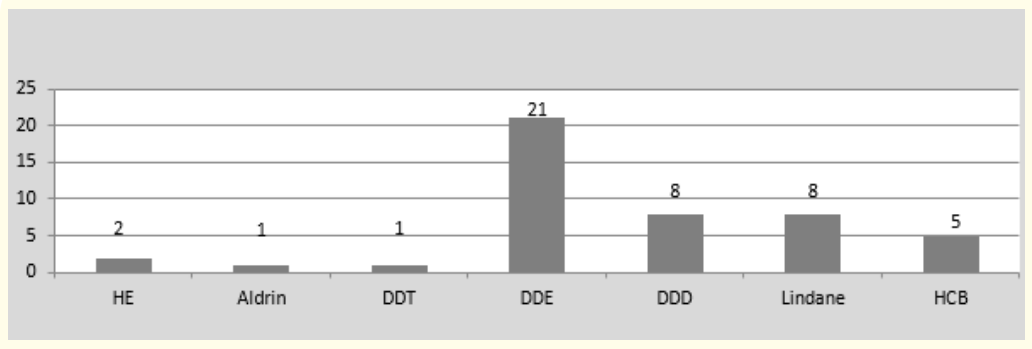
Figure 3: Number of samples contaminated with each pesticide.
The metabolism of organic pollutants generally results in the passage from the lipophilic to the hydrophilic form, which can be eliminated by the kidneys. The levels of these toxins in breast milk are therefore dependant on maternal metabolism. In some cases, however, the metabolite also accumulates in breast milk. This is the case of DDT metabolized to DDE aerobically and to DDD anaerobically [36]. Its half-life in soil is 15 years. The presence of these metabolites in the absence of DDT in 24 of the 25 samples tested positive is not in favor of a recent use. However, the latter is not totally excluded since DDT was found in a single sample and at levels exceeding the MRL for total DDT. A study conducted by the American University of Beirut (AUB) and the IDRC (International Development Research Center - Canada) in 2003 confirmed the presence of DDT and its metabolites in the soil (in southern Lebanon and in particular in Tyre) and in water (Al Kabir river in North-Lebanon) [37].
In our study the levels of pesticides exceeded the MRLs in 10 samples (16.7%) (Figure 4). The most toxic levels are those of Lindane: 7 out of eight positive samples (87.5%) exceeded the MRL. Lindane is a derivative of HCB. It has been used since the 1940s as a pesticide in agriculture and for the treatment of lice and scabies in humans. Lindane is not part of the list of POPs subject to the Stockholm Convention [7] and its extensive use persists in many countries. It is responsible for the contamination of essential food products during childhood [38]. With PBDEs (Polybrominated Diphenyl Ether - PCB analogues and flame retardants), nitro-musk derivatives (used in the composition of perfumes, soaps and detergents), PFOA and PFOS (Perfluorooctanic Acid and Perfluorooctane Sulfonate respectively - synthetic surfactants found in lubricants, paints, cosmetics, the coating of non-stick pans and food packaging, Lindane is part of a new class of so-called ‘’emerging’’ POPs [39]. Currently the Food and Drug Administration (FDA) recommends caution regarding its use because of potential neurological toxicity [40].
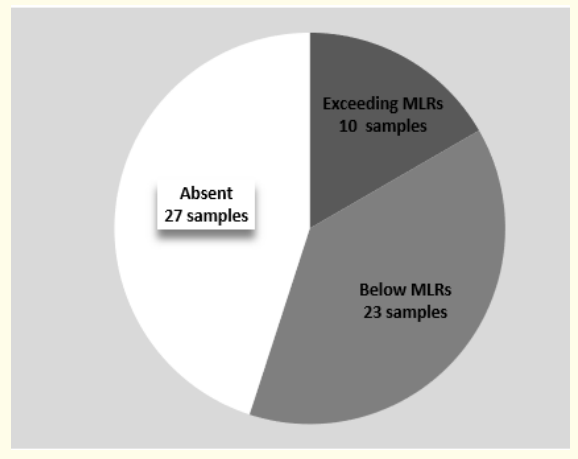
Figure 4: Distribution of pesticide concentrations in the breast milk of the study population.
In the literature, age is the demographic factor that most influences the levels of organochlorine pesticides in breast milk [4143]: with age, body fat increases, facilitating the accumulation of lipophilic toxins. They also depend on the level of lipids in breast milk, and the body mass index: the higher the milk lipids and BMI, the lower the concentration of chemical pollutants [36]. This could partially be explained by a dilution effect. In addition, smoking and alcohol consumption enhances the concentrations of most toxins in breast milk [41].
Infant exposure begins in the antenatal period, well before lactation. Breastfeeding, however, appears to be the only way of eliminating pesticides from the maternal body [44]: the first born would therefore be exposed to the toxins accumulated by his mother during her entire life and children breastfed for 6 months have been shown to accumulate 14% of their pesticide load [45]. In our study, no relationship was demonstrated between organochlorine pesticide levels and age, BMI, maternal smoking, parity and breastfeeding duration. However, the highest DDT levels were observed in a sample obtained from a 28-year-old primiparous woman with a BMI of 18.8 who had been breastfeeding for only one month. In fact, food is the main source of exposure to POPs [46] and our results confirm data from literature. It is, above all, the consumption of fatty foods of animal origin that contributes to their human burden [29,37]. Between 1984 and 1988, a study conducted by the National Council for Marine Sciences found high levels of DDE and DDT in two species of fish highly prized by Lebanese [37]. Data available in 2000 shows the persistence of these pesticides at comparable rates [47]. In the late nineties similar results were reported in Lebanon by Dagher for fatty meat and fish consumption [3] and several authors have reported a positive correlation between fish consumption and DDE levels in breast milk [48] and plasma [49].
Data from literature show an association between high levels of contaminants in the mother’s blood and negative effects on development in infants [15-17]. Even a slightly higher than the average exposure can have long-term consequences on their cognitive development. However the studies conducted have mainly focused on prenatal exposure to POPs. While some show a positive correlation between the levels of POPs in breast milk and low cognitive indices [12,15] they also show that breastfed children have better neurological scores than those receiving infant formula, regardless of the levels of POPs in breast milk [12]. Furthermore, pesticide levels in breast milk may only reflect earlier in utero exposure. Thus, with respect to potential adverse health effects, the role attributed to in utero exposure to POPs appears to be more important than the role of lactational exposure. If potential adverse effects are balanced against positive health aspects for breastfed infants, the advantages of breastfeeding far outweigh the possible disadvantages.
Our observations provide a strong argument to plea for further global source-directed measures to reduce human exposure. At the national level, prevention is based on the application of the laws in force prohibiting the use POPs. Additional efforts must be made in the inspection of storage sites and points of sale of these products and in the control of landfill sites. Awareness programs must be extended to the general population and to farmers who sometimes lack information concerning prohibited products, their health effects and the methods of contamination. Many emerging pollutants are currently the subject of increased media coverage. These new molecules raise concerns related to their potential toxicity. These concerns are justified by structural and functional similarities to other POPs and by similar characteristics of bioaccumulation and trans-placental transfer. Their health effects in humans have not yet been proven, but past experience with PCBs, dioxins and DDT can only lead to caution about their use. Pending the true application of the measures prohibiting their use, exposure to POPs should be limited when possible by early introduction of dietary habits limiting the consumption of fatty foods of animal origin.
Breast milk is more subject to environmental contamination than infant formula: its dynamic composition, which is one of its main assets, depends on the accumulation of lipophilic toxins in maternal adipose tissue. Numerous studies have shown a positive association between exposure to organochlorine pesticides and adverse effects on child development and health. The respective contribution of intrauterine exposure and breastfeeding to its health effects are not yet known. Currently, breast milk remains the nutrient of choice for newborns under 6 months of age: its beneficial effects are clearly greater than the potential risks associated with its contamination. Our results suggest a non-negligible contamination of the milk of Lebanese women by organochlorine pesticide residues. The most frequently found pesticides are DDE and Lindane. Admittedly, DDE levels are lower than those reported in 1999, but recent contamination does not seem to be excluded and the MRLs of the pesticides studied are exceeded in 16.7% of the samples. The most toxic levels are those of lindane. Dosage of POPs in breast milk or maternal blood is not recommended in the general population. The tests are expensive and very difficult to interpret. Given their specific characteristics (persistence, bioaccumulation, lack of means of elimination), the only way to reduce their health effects is to limit exposure. This first requires the application of the laws in force prohibiting their use. The information of women of childbearing age on the potential sources of contamination by POPs seems essential. It prevents unwarranted fears about breastfeeding and advocates good health habits across generations. In the populations most at risk, advice on feeding habits must be given as early as possible and begin in childhood: it is the only way to allow women to reach the age of procreation with the minimum of foeto-toxic substances in their body.
Our study is a cross-sectional study, but it is the first study measuring the contamination of breast milk with organochlorine pesticides carried out in Lebanon since 1999. Since pesticide levels are dependent on lipid levels in breast milk, it would have been preferable to express our results in micrograms/g of lipids in breast milk. By way of comparison, it would also have been desirable to measure the levels of organochlorine pesticides in samples of infant milk present on the Lebanese market.
Copyright: © 2022 Fares Florence. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.