Dr. Krishna Kumar M1, Dr. Vishwanathan M2. Dr. Anand Kumar .B3, Dr. Senthilnathan.V1. Dr. Rajarama Ravi Varma.N3
1 Professor of Radiology, Department of Radio-diagnosis. Trichy SRM Medical College Hospital & Research Centre. Irungalur, Trichy, India
2 Postgraduate Student and Junior Resident, Department of Radio-diagnosis. Trichy SRM Medical College Hospital & Research Centre. Irungalur, Trichy, India
3 Senior Resident, Department of Radio-diagnosis. Trichy SRM Medical College Hospital & Research Centre. Irungalur, Trichy, India
*Corresponding Author: : Krishna Kumar M, Professor of Radiology, Department of Radio-Diagnosis, Trichy SRM Medical College Hospital and Research Centre, Irungalur, Trichy, India.
Received: February 16, 2022; Published: March 30, 2022
Citation: Krishna Kumar M., et al. “Primary Endobronchial and Mediastinal Synovial Sarcoma in a Young Female”. Acta Scientific Paediatrics 5.4 (2022): 32-37.
Primary thoracic synovial sarcomas (SS) typically occur as chest wall masses, although they rarely arise in the lung and pleura. Primary pulmonary and mediastinal SS is very uncommon in comparison with metastatic sarcoma and might arise in the tracheobronchial tree manifesting as an endoluminal mass, generally in adults. Signs and symptoms may additionally consist of wheezing, persistent pneumonia, bronchial asthma, chest ache, recurrent cough, atelectasis, hemoptysis, and weight loss. Because of the heterogeneity of signs and symptoms, clinical diagnosis may be tough. Herein, we present a case of primary endobronchial and mediastinal SS in a young female presenting with cough and expectoration associated with haemoptysis and breathlessness on exertion for 1 week with particular emphasis on the radiologic and pathologic findings of this rare lesion.
Keywords: Endobronchial Tumour; Mediastinal Tumour; Pulmonary Synovial Sarcoma; Primary Pulmonary and Mediastinal Synovial Sarcoma; Small Round Cell Tumour; Tracheobronchial Tumour.
Primary tracheobronchial tumors are rare lesions which may be benign or malignant with different positions along the airway tree. Primary malignant tumors in the tracheobronchial tree are uncommon, accounting for lesser than 1% of all thoracic malignancies [1]. Primary pulmonary SS is very rare compared with metastatic sarcoma, accounting for less than 0.5% [2]. Primary pulmonary and mediastinal SS is an aggressive tumor sharing not unusual histological features with soft tissue synovial sarcoma [3,4]. Despite the fact that, the ideal histogenesis of primary pulmonary SS is uncertain and could origin in pleuripotent mesenchymal cells of bronchial submucosal stromal tissue [5]. Molecular testing for the pathognomonic t(x;18) chromosomal translocation has enabled diagnostic confirmation in approximately ninety percent of instances. In t(x;18)-negative cases, diagnosis must relay on histological and immunophenotypic capabilities [6].
Endobronchial and endotracheal tumors within the pediatric population are more likely to be malignant instead of benign [7]. Primary malignant tumors within the tracheobronchial tree can produce signs and symptoms of airway obstruction (dyspnea, wheezing, stridor), mucosal infection and ulceration (cough, hemoptysis), or involvement of adjacent structures ( recurrent laryngeal nerve palsy & dysphagia) by direct invasion [8,9].
We evaluated the clinical, radiological and pathological findings in young female of primary endobronchial and mediastinal synovial sarcoma.
15 years old young girl presented to the respiratory medicine department a tertiary care hospital with complaints of Cough and expectoration associated with haemoptysis and breathlessness on exertion for 1 week. The blood examination showed mildly elevated lymphocytes (45.3%) and monocytes (13.1%). Blood haemoglobin was within normal limits. A provisional diagnosis of Adenocystic carcinoma / Carcinoid was made based on the clinical findings.
Bedside Chest X-ray Antero-posterior view taken shows right lung partial Consolidation collapse and mediastinal shift to the right side (Figure 1).
Patient referred for Multidetector Computed Tomography (MDCT) Chest revealed a well defined multi-lobulated heterogeneously enhancing lesion in the carina measuring ~ 23 x26 mm, extending to the right lower lobe bronchus with significant extraluminal component causing abrupt cut off of the bronchus intermedius causing lower lobe collapse (Figure 2-4). The extraluminal component measuring 44 x 38mm is abutting the 5th costovertebral junction with no obvious erosion or scalloping (Figure 2-4) - features are likely of neoplastic etiology. Differential diagnosis include neuroendocrinal tumor versus germ cell tumour.
Computed Tomography Virtual Bronchoscopy images (Figure 5) exhibit lobulated tumor arising from the right main bronchus, occluding lumen, projecting into the carina & lower trachea. Metastatic SS to the lung (Figure 6) became excluded with an intensive clinical and radiological examination to exclude primary in the body.
The Patient underwent Rigid Bronchoscopy which showed broad based lobulated mass arising from the posterior wall of trachea extending to the right main bronchus causing a complete luminal obstruction. Endobronchial mass debulking become accomplished with electrocautery snare and specimen sent for Histopathological exam (HPE).
HPE demonstrated fragments of bronchial mucosa with subepithelial diffuse infiltration by small round cells having a scanty amount of cytoplasm and hyperchromatic nuclei. Many interspersed thin walled blood vessels are present. Findings were suggestive of Malignant Small Round cell tumour. Immuno-histochemistry (IHC) was done for definite cell typing. The neoplastic cells were negative for LCA, Pancytokeratin, Synaptophysin, S100, Desmin and positive for CD 99, BCL2, Vimentin, EMA and CD 56 with an MIBI index of 80%.. The findings favoured the diagnosis of poorly differentiated synovial sarcoma (SS). The patient was advised Translocation studies for confirmation and further management at a better centre of excellence.
Patient was advised Translocation studies for confirmation, but patient was lost for follow-up.
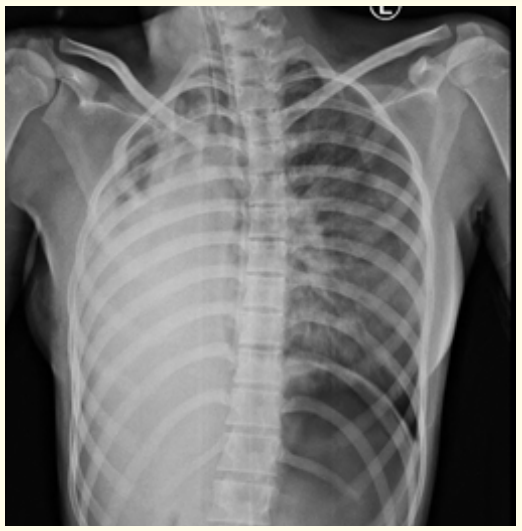
Figure 1: Chest X-ray Antero-posterior view depict Right lung partial Consolidation collapse and mediastinal shift to the right side.
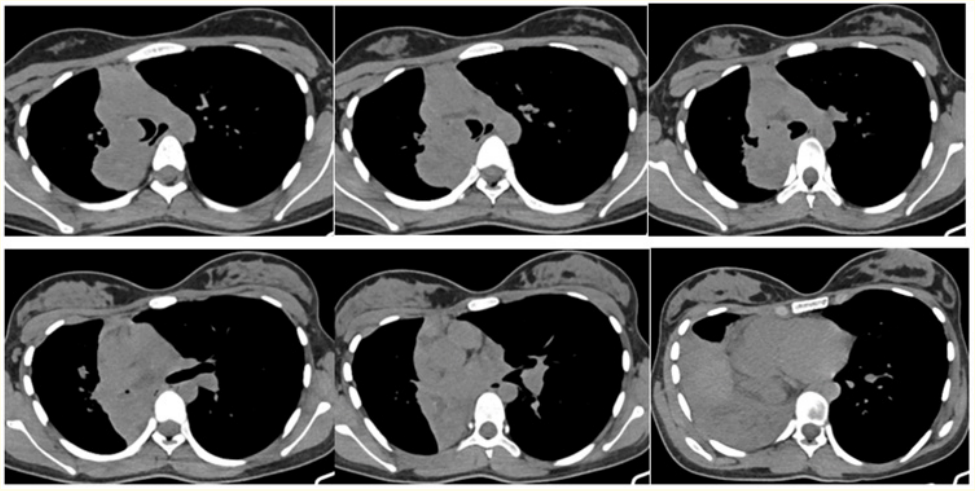
Figure 2: Axial (A-F) Non-contrast Computed Tomography images show a multilobulated isodense mass in the distal trachea extending to the right lower lobe bronchus with significant extraluminal component causing abrupt cut off of the bronchus intermedius causing lower lobe collapse.
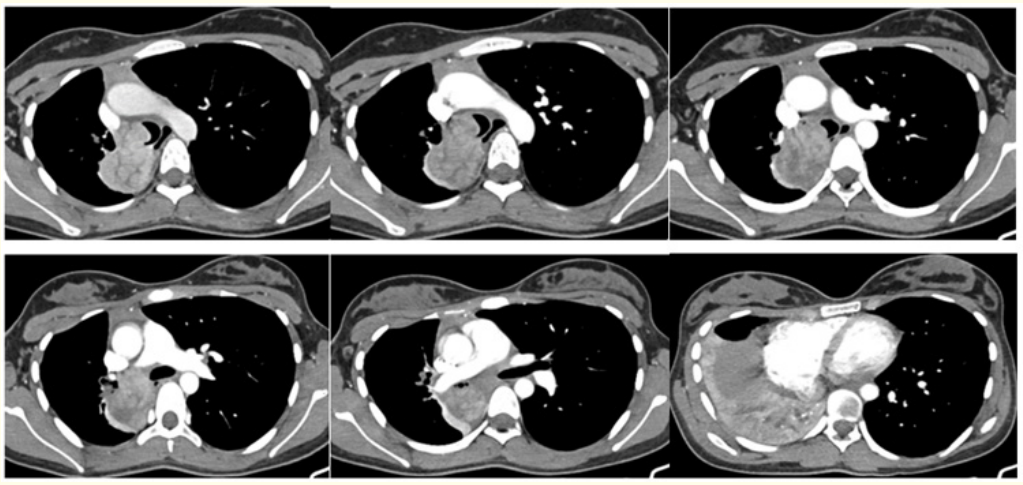
Figure 3: Axial (A-F) Contrast enhanced Computed Tomography images show well defined multi-lobulated heterogeneously enhancing lesion in the distal trachea, carina extending to the right lower lobe bronchus with significant extraluminal component causing abrupt cut off of the bronchus intermedius, causing middle lobe & lower lobe consolidation collapse.
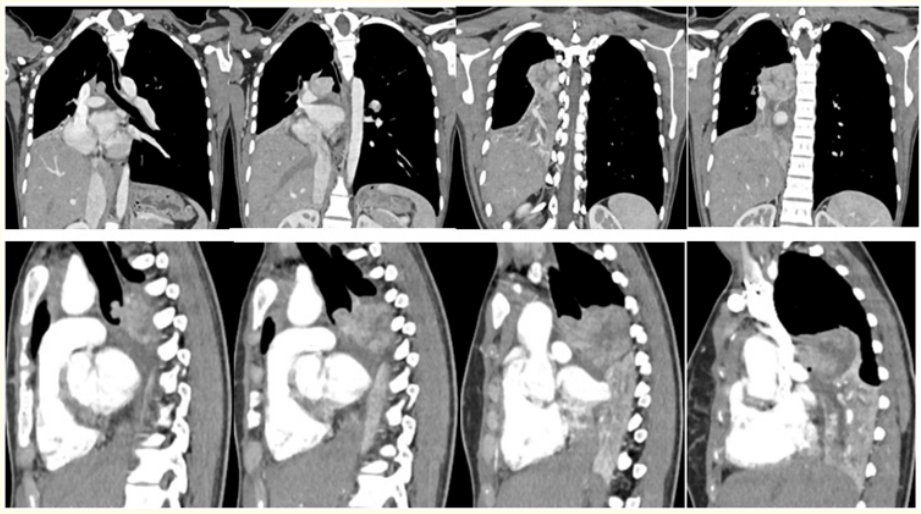
Figure 4: Reconstructed Coronal (A-D) and Sagittal (E-H) Contrast enhanced Computed Tomography images reveal multilobulated endotracheal and right endobronchial tumour occluding the right main brochus
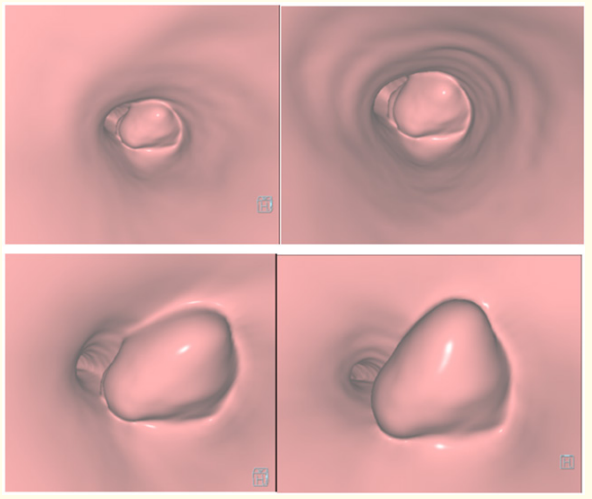
Figure 5: Computed Tomography Virtual Bronchoscopy (A-D) images exhibit lobulated tumor arising from the right main bronchus, occluding lumen , projecting into carina & lower trachea.

Figure 6: Axial (A-E) Computed Tomography lung window images reveal endobronchial and mediastinal mass with no obvious metastasis.
Maximum primary pulmonary and mediastinal synovial sarcomas are located within the lung parenchyma [3, 4] and rarely extend into the bronchial tree [10] or occur inside the heart or pericardium [4]. Findings from 5 earlier series [3, 4, 10] suggest that, in contrast to soft tissue synovial sarcoma, primary pulmonary and mediastinal synovial sarcoma happens in older patients without gender bias. Results from five prior series [3, 4, 10] indicate that, in contrast to soft tissue synovial sarcoma, primary pulmonary and mediastinal synovial sarcoma occurs in older patients without gender bias. Clinical symptoms are site specific with few asymptomatic instances [4]. In contrast our case of primary endobronchial and mediastinal SS occurred in young female, causing cough, hemoptysis and breathlessness on exertion.
Primary pulmonary SS shares similar histomorphological and chromosomal translocations t(X; 18) as its soft-tissue counterpart [10, 11]. Histologically, primary pulmonary and mediastinal synovial sarcoma reportedly shares similar findings with soft tissue synovial sarcoma including dense cellularity, interlacing fascicles, hyalinized stroma, hemangiopericytoma like vasculature, focal myxoid exchange, and mast cellular influx [10]. In our study, the HPE was suggestive of malignant small round cell tumour and immunohistochemistry (IHC) features consistent with poorly differentiated synovial sarcoma (SS). Begueret et al [4] stated that almost forty percent of primary pulmonary and mediastinal synovial sarcomas have been poorly differentiated, which is similar to our case study.
The term “Small Round Cell Tumours” (SRCT) applies to a cluster of extraordinarily aggressive malignant neoplasms which feature the predominantly small and monotonous undifferentiated cells with extensive nucleocytoplasmic ratios on histology. This group includes Ewing’s Sarcoma (ES), Primitive Neuroectodermal Tumour (PNET) or extraskeletal Ewing’s sarcoma, neuroblastoma, rhabdomyosarcoma, desmoplastic small round cell tumour, non Hodgkin’s lymphoma, small cell osteosarcoma, small cell carcinoma (either undifferentiated or neuroendocrine), olfactory neuroblastoma and mesenchymal chondrosarcoma. Their clinical presentations frequently overlap, for that reason making the diagnosis difficult in some instances. Within the recent past, more and more sophisticated array of immunohistochemical and chromosomal markers have confirmed to be beneficial in classifying these aggressive lesions [12].
Although immunohistochemistry (IHC) may be a useful adjunct, it lacks specificity. Amongst several histological variants of SS, monophasic fibrous subtype is difficult to diagnose and desires to be differentiated from sarcomatoid carcinoma, sarcomatoid variation of mesothelioma, fibrosarcoma, leiomyosarcoma, Ewing’s sarcoma, spindle cell thymoma, and solitary fibrous tumor (SFT) in addition to metastatic sarcomas [2-5]. Therefore the utility of fluorescent in situ hybridization (FISH) strategies to detect particular places similar to the lung [2,4].
SSs are characterized by way of the particular chromosomal translocation t(X;18) (p11.2;q11.2), which has been detected in more than ninety percent of SS [13]. It finally ends up in the fusion of the SYT gene from chromosome 18 to 1 of three exceptionally homologous genes at Xp11, namely, SSX1, SSX2, and in rare instances, SSX4 [13,14].
On chest roentgenogram, the lesion is often uniform with wellcircumscribed rounded or lobulated borders [15–17] with a mediastinal shift in some patients [18].
CT shows a well-defined homogeneous or heterogeneously enhancing mass containing necrotic areas and soft tissue components. Ipsilateral pleural effusion is common [15–18], while mediastinal lymphadenopathy is rare [15].
Totally based on 2-year local recurrence quotes [20, 21] and poor five-year disease-specific survival rates [4], primary pulmonary and mediastinal SS is greater aggressive than soft tissue synovial sarcoma. Primary pulmonary SS behaves more aggressively due to the late presentation, massive tumor size and trouble in reaching free surgical margins.
Free surgical margins and adjuvant chemotherapy increases the time for nearby recurrence and sickness-free survival. Although, the prognosis of primary pulmonary SS stays poor with overall survival of 50% [19]. the prognosis of primary endobronchial SS subset isn't always defined. The prognosis in our case study couldn’t be predicted because the patient was lost for follow up. .
The Majority of primary pulmonary and mediastinal SS are located within the lung parenchyma and barely extend into the bronchial tree. Because of the heterogeneity of symptoms, clinical diagnosis is difficult. Cross sectional CT imaging is crucial to grasp the precise location & extent of tumour. Histologically, primary pulmonary and mediastinal SS reportedly stocks similar features with soft tissue SS. Within the latest past, more and more sophisticated array of immunohistochemical and chromosomal markers have proven to be useful in classifying those aggressive lesions.
Copyright: © 2022 Krishna Kumar M., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.