Ana de Carvalho Vaz1*, João Oliveira2, Diana Pinto3, Fernanda Teixeira3, Ana Rita Araújo3 and Laura Marques4
1 Department of Pediatrics, Unidade Local de Saúde do Alto Minho, Viana do Castelo, Portugal.
2 Department of Pediatrics, Centro Materno Infantil do Norte, Centro Hospitalar Universitário do Porto, Porto, Portugal
3 Department of Pediatrics, Pediatric Alergoly Unit, Centro Materno Infantil do Norte, Centro Hospitalar Universitário do Porto, Porto, Portugal
4 Department of Pediatrics, Infectious Diseases and Immunodeficiencies Unit, Centro Materno Infantil do Norte, Centro Hospitalar Universitário do Porto, Porto, Portugal
*Corresponding Author: Ana de Carvalho Vaz, Department of Pediatrics, Unidade Local de Saúde do Alto Minho, Viana do Castelo, Portugal.
Received: March 15, 2022; Published: March 25, 2022
Citation: Ana de Carvalho Vaz., et al. “Dupilumab Successful Treatment of a Child with Severe Atopic Dermatitis”. Acta Scientific Paediatrics 5.4 (2022): 19-23.
Poorly controlled moderate-to-severe atopic dermatitis in children and adolescents can result in growth failure, impaired quality of life of patients and their families and a significant increase in healthcare expenses. A 9-year-old boy with severe atopic dermatitis, who was initiated on off-label treatment with dupilumab and presented a significant improvement in dermatitis and quality of life.
This case highlights the great potential of dupilumab in the treatment of severe atopic dermatitis in the pediatric population.
Keywords: Dupilumab; Atopic dermatitis; Quality of life; Eczema; Children.
AD: Atopic Dermatitis; EASI: Eczema Area and Severity Index; CDLQI: Children Dermatology Life Quality Index; SCORAD: SCORing Atopic Dermatitis ; QoL: Quality Of Life
Atopic dermatitis (AD) is one of the most common skin disorders [1], affecting approximately 10-20% of the pediatric population. It is estimated that at least one third of these patients present with moderate-to-severe disease [2].
In most patients with mild disease, treatment goals are achieved with topical therapies alone. In moderate-to-severe cases, the management and treatment of the disease is challenging [2]. For decades, the treatment of severe AD has focused on the use of topical and non-specific systemic immunosuppressive agents, often with ineffective results [3,4]. Due to the risk of long-term complications, especially in the pediatric population, some therapeutic choices might not be appropriate for prolonged maintenance therapy [2] and most remain as off-label treatments [2,5].
Recently, the introduction of novel systemic treatments, such as interleukin and JAK inhibitors have expanded the therapeutic portfolio of systemic treatments in adults. Therefore, a considerable number of biologics are currently being investigated in the pediatric population.
Dupilumab, a fully humanized monoclonal antibody that inhibits the signaling of interleukin IL-4 and IL-13, reducing type 2 inflammation [6], was the first and only targeted systemic treatment licensed to treat moderate-to-severe AD. It was first approved for adults in March 2017 and 2 years later for adolescents aged 12 years and older [7]. In several phase 3 clinical trials, dupilumab proved to be safe and effective in treating moderate-to-severe AD in patients aged ≥ 12 years and in patients with severe AD as young as 6 years [7,8].
An 8-years-old Caucasian boy with severe AD since early childhood was referred to our tertiary center. He was born to a nonconsanguineous parents, with both father (rhinitis) and brother (food allergy and rhinitis) with a history of atopia. The parents reported that dermatitis began at three months of age. AD was never adequately controlled, despite appropriate emollient therapy, skin care, and numerous topical anti-inflammatory therapies.
At age 5, AD worsened, and several therapies were prescribed: topical corticosteroids and short courses of systemic corticosteroids, topical pimecrolimus, systemic Montelukast, and antihistamines. Nevertheless, he presented with skin infections managed with multiple courses of topical and systemic antibiotics and required two hospitalizations for intravenous antimicrobial and corticosteroid treatment. By the age of 6 years, he presented with persistent allergic rhinitis, and laboratory assessment revealed eosinophilia, elevated IgE levels (3090 KU/L), and polysensitization to food and inhalant allergens. His rhinitis improved significantly after a three-year course of mite immunotherapy, but on the contrary, his eczema was not controlled, despite multiple foods avoidance (milk, egg and wheat). A 3-month trial with oral cyclosporine was attempted, but no clinical improvement of the eczema was observed.
The referral to our center at the age of 8 years was followed by an analytical and clinical worsening figure 1. During follow-up at our center, the patient’s serum IgE ranged from 2205 to 7020 KU/L. He had normal IgG, IgA, and IgM levels, and normal responses to protein and polysaccharide-based vaccines. Primary immunodeficiencies were excluded, including DOCK 8 and STAT3 deficiency. Filaggrin deficiency was also excluded. Serum protein electrophoresis showed no monoclonal bands and Th17-cell counts in the peripheral blood were normal.
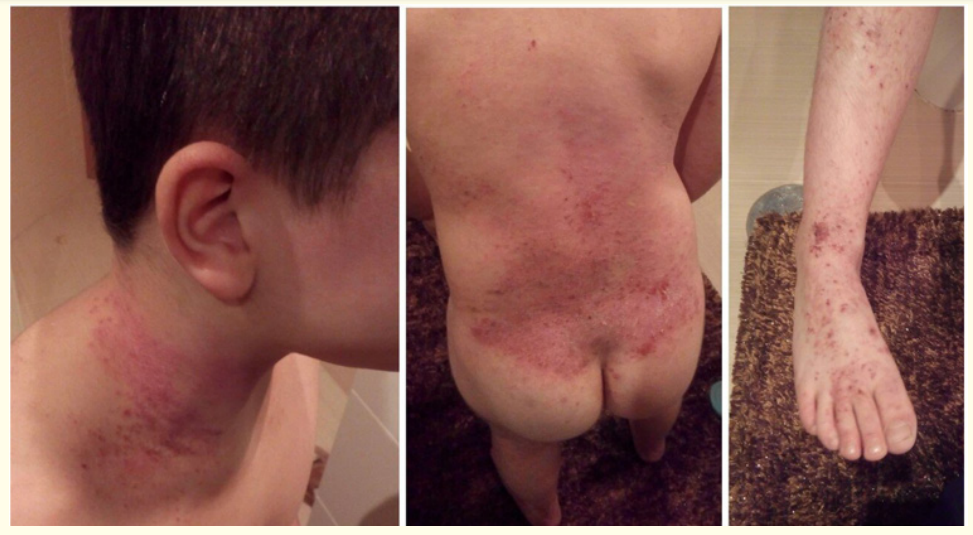
Figure 1: Excoriated, erythematosous and eczematous plaques scattered in the neck, trunk and feet, before dupilumab treatment.
The patient’s dermatitis severity was evaluated using the “Scoring of Atopic Dermatitis” (SCORAD) (total score 52) and the “Eczema Area and Severity Score” (EASI) (total score 22), revealing severe disease with a profound effect on quality of life (QoL), assessed by the “Children´s Dermatology Life Quality Index” (CDLQI) (total score of 25).
He was started on systemic corticosteroids (1mg/kg/day) and his symptoms improved, which translated into an improved SCORAD score, which dropped to 37. Despite perceived improvement, his disease often flared up and the side effects of long-term corticosteroids became apparent, with a cushingoid appearance and weight gain. Moreover, the patient started to present with worrisome comorbidities, such as learning difficulties, sleep disturbances, and behavioral disorders, and was referred to a psychology consultation.
Considering the severe course of the disease, despite aggressive topical and systemic therapies, and the long-term adverse effects of corticosteroids, and the comorbidities observed, at the age of 9, the patient was started on off-label dupilumab, after approval of the Pharmacy and Therapeutics Committee and Ethics Commission of our center.
Written informed consent was obtained from his parents. The patient received a subcutaneous injection of a loading dosage of 600 mg of dupilumab, followed by 300 mg every month, with the assumption of future adjustments based on his response to therapy.
Treatment response was assessed based on SCORAD and improvement in QoL was assessed using CDLQI, with the questionnaires being completed by the patient and by their parents, prior to dupilumab treatment and every 2 weeks.
The patient´s eczema and pruritus improved significantly within 6 weeks of the first dose of dupilumab, resulting in improved sleep and QoL, with decreases in SCORAD (total score 20,1) and CDLQI (total score 6). After the second dose of dupilumab, the patient was able to progressively reduce systemic steroids that were discontinued 12 weeks after the biological agent was introduced.
Eight months after dupilumab´s therapy, the patient presented with palpebral eczema and lesions on the neck, despite concomitant treatment with topical corticosteroids, and the dupilumab dose was increased to 300 mg once every 2 weeks. During treatment, the patient was monitored for eye symptoms, and he was started treatment on topical eye drops, after a mild transitory conjunctivitis, which led to a reduction in the dupilumab dose to 300 mg once every 3 weeks, a dose regimen that he currently maintains.
Twenty-four months after starting treatment, at 11 years of age, the patient continues to tolerate dupilumab with a sustained response. He currently presents with only mild palpebral dermatitis, which is controlled with topical corticosteroids, and no other skin lesions on visual examination figure 2. The evolution of the SCORAD, EASI, and CDLQI scores during management is shown in figure 3. Twenty-four months after treatment initiation, the SCORAD score is 10 and the EASI and CDLQI scores are 1. Additionally, a marked decrease in total IgE (349 KU/L) and specific IgE levels was observed. He currently follows a well-balanced diet, without any food restrictions. When comparing evaluations before and 24-months after dupilumab initiation, weight reduction from the > 97th percentile to the 85-97th percentile and an improvement in statural growth were observed, with height moving from the 10th percentile to the 25th percentile.
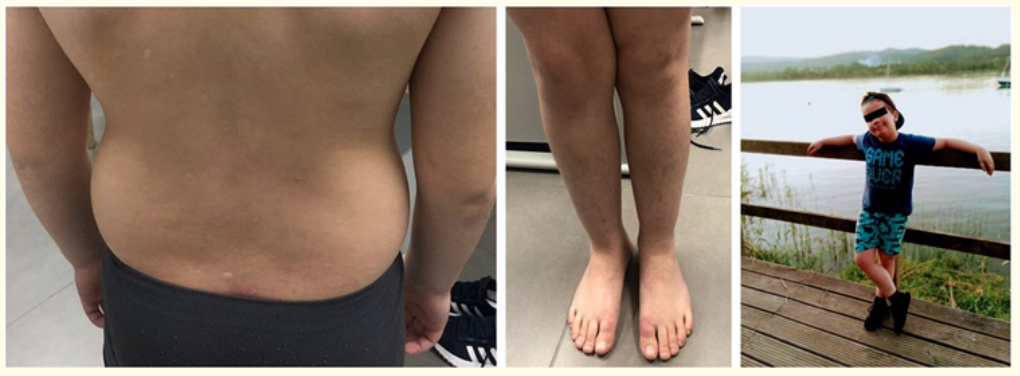
Figure 2: Patient after dupilumab treatment, showing dramatically improvement in his eczema.
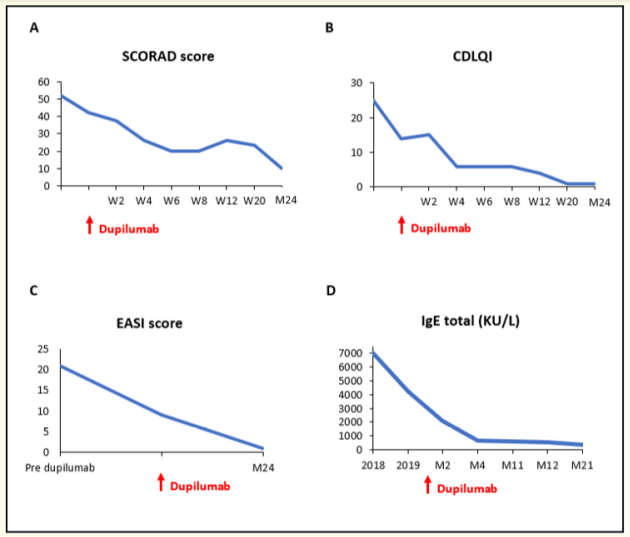
Figure 3: A, B: Standardized Scoring of Atopic Dermatitis (SCORAD) and Children´s Dermatology Life Quality Index (CDLQI) scores at dupilumab initiation and at multiple time points on treatment. C: Eczema Area and Severity Score (EASI) score before, at dupilumab initiation and at month 24. D: Serum IgE levels before dupilumab initiation and at multiple time points on treatment.
Our case report describes a patient with severe AD and elevated IgE levels, who had a rapid and sustained improvement of his eczema, including itch, sleep, and QoL, with near-complete regression of lesions within 12 weeks after the introduction of dupilumab therapy.
The treatment response observed in our patient was not significantly different from that reported in the adult and adolescent trials. SCORAD, EASI, and DLQI scores showed sustained improvement after 24 months of treatment. We also observed a substantial decrease in IgE levels and allergen-specific IgE concentration and a significant decrease in skin infections.
New targeted therapies for AD have been developed based on an increased understanding of the disease pathogenesis. Dupilumab was the first approved monoclonal antibody targeting the shared IL-4Rα subunit that inhibits IL-4 and IL-13 signal transduction and aberrant Th2 responses [9].
In this case report, dupilumab was used off-label since it had only been approved for treatment of moderate-to-severe AD in patients aged 12 years or older [9]. Patient dosing parameters were based on ongoing dupilumab clinical trials in the pediatric population [10] and adjustments were based on patient clinic response and adverse effects. Ophthalmic adverse events have been reported in a minority of patients in clinical trials [9] and our patient developed mild conjunctivitis, after increasing the dupilumab dose 300 mg every 2 weeks, leading us to hypothesize that this interval between doses was too short.
Although dupilumab does not require routine laboratory monitoring [10], laboratory tests were performed 1, 3, 6 and 12 months after dupilumab initiation and revealed no relevant toxicity.
At the time of manuscript submission, dupilumab had already been licensed for the treatment of moderate-to-severe AD in patients aged ≥ 6 years.
Dupilumab was a very important addition to AD therapies available for children with moderate-to-severe disease, who are candidates for systemic therapy. Given the physical, emotional and psychological comorbidities of this condition, patient selection should be individualized and barriers to its use should be minimized. Pediatric patients deserve timely access to new treatments given the potential impact of AD on growth and development, socialization, school performance and direct and indirect healthcare costs [9,10].
Our patient´s results suggest that dupilumab is a satisfactory therapy, not only in terms of disease activity control but also in terms of its impact on quality of life, with an acceptable safety profile, over a longer duration of treatment (at least 24 months), which is consistent with recent clinical trials that have been conducted.
It is crucial to make a concerted effort to optimize the use of new therapies, such as dupilumab, in AD patients and possibly, in other related diseases involving type 2 inflammation [10].
Authors state no conflict of interest.
Copyright: © 2022 Ana de CarvalhoVaz., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.