Liliana Sá*, Ana Maria Ferreira, Teresa Pinheiro and Teresa Caldeira
Centro Hospitalar de Entre o Douro e Vouga (CHEDV), Department of Paediatrics and Neonatology, Portugal.
*Corresponding Author: Liliana Sá, Centro Hospitalar de Entre o Douro e Vouga (CHEDV), Department of Paediatrics and Neonatology, Portugal.
Received: December 24, 2021; Published: January 31, 2022
Citation: : Liliana Sá., et al. “Prenatal and Early Postnatal Diagnosis of Congenital Toxoplasmosis: Retrospective Study”. Acta Scientific Paediatrics 5.2 (2022): 30-36.
Background: Congenital toxoplasmosis occurs when there is a maternal primary infection during pregnancy and transplacental transmission of the parasite occurs to the fetus. The incidence is about 1- 10/10.000 births. It is a preventable disease with possible severe consequences. In the postnatal period, diagnosis is established by the persistence of anti-Toxoplasma IgG by 12 months of age.
Methods: We performed a retrospective study of children with suspected congenital toxoplasmosis born in our hospital between 2008 and 2018. The aim was to define the clinical and serological characteristics of children with congenital toxoplasmosis to optimize diagnostic work-up and treatment.
Results: Of the 51 suspected cases only 7.8% proved to be infected. Seroconversion occurred mostly in the 1st trimester (49%). Amniocentesis was performed in 39.2% of women and all tested negative in the polymerase chain reaction for Toxoplasma gondii DNA detection in the amniotic fluid. 60.8% of women were treated with spiramycin. All children were asymptomatic at birth except for one that presented with chorioretinitis. 68.6% of infants started treatment immediately after birth (40% spiramycin, 57% pyrimethamine/sulfadiazine). Median treatment duration was 2.4 months and interruption was determined by a negative polymerase chain reaction for Toxoplasma gondii in peripheral blood. None tested positive for toxoplasma specific IgM nor polymerase chain reaction test. In two cases anti- Toxoplasma IgG tested positive after the first year of life and treatment was re-introduced. No significant impairments were detected.
Conclusions: Given the characteristics of this infection, to confirm or exclude congenital toxoplasmosis several serological and parasitological tests are required.
Keywords: Chorioretinitis; Congenital Infection; Congenital Toxoplasmosis; Thalamic Calcifications; TORCH.
CT: Congenital Toxoplasmosis; PCR: Polymerase Chain Reaction; AF: Amniotic Fluid
Toxoplasmosis is a global health problem. This zoonotic disease is spread worldwide and is caused by Toxoplasma gondii (T. gondii), an obligate intracellular protozoan that infects humans by ingestion of oocysts that contaminate food, water and soil. In the acute phase of the disease, the parasite in its tachyzoite stage replicates and disseminates from cell to cell. In this stage they it can cross the placenta and infect the fetus. Later, tachyzoites differentiate into bradyzoites that form tissue cyst, predominantly in the central nervous system, eye and muscle, where they may rest for the lifelong of the host [1]. Toxoplasmosis is usually asymptomatic in immunocompetent subjects. However, T. gondii infection acquired during pregnancy can lead to fetal infection. Congenital toxoplasmosis (CT) may result in intrauterine death, symptomatic newborns or newborns with nonspecific symptoms at birth. Fetal damage usually involves the brain and the eyes [2-4], and unless treated, visual or neurological sequelae may develop later in life [4].
In Portugal there is a National Universal Screening Program that includes the screening of anti- Toxoplasma IgM and IgG in each trimester, to detect maternal seroconversion during pregnancy. Our estimated seroconversion rate is 1.7:1000 [5]. CT affects one to ten fetuses per 10 000 live born infants with a well-known south to north geographical gradient in Europe [6]. In France some studies report an incidence rate of acute primary T. gondii infection of 2.1 in 1000 in pregnancies, an estimated CT prevalence of 3.3 per 10 000 live births and a rate of 0.3 per 10 000 live births for symptomatic CT [7].
Gestational age at the time of maternal infection is known to have a marked impact on the rate of clinical findings in infected infants. The earlier the infection occurs during pregnancy, the higher the likelihood of clinical signs but if the infection occurs later in pregnancy, infected infants have higher probability of a more favorable prognosis [8]. The risk of fetal infection increases from less than 10% in the first trimester, 30% in the second and 70% to 90% in the third trimester. Infection acquired up to 2 months before conception may also endanger the fetus [9,10].
Congenital toxoplasmosis has a broad spectrum of nonspecific clinical manifestations. The so-called classic triad of CT consists of chorioretinitis, hydrocephalus and intracranial calcifications. However, most newborns with CT are asymptomatic and the classic triad occurs in fewer than 10 percent of the cases [11].
Frequency of severe congenital infection can be limited by routine screening of mothers and babies and by early specific treatment [12,13]. Preventive strategies like restraining from handling cat litter or raw meat, eating undercooked meat, carefully wash fruit and vegetables, or gardening, are routinely counseled to nonimmune pregnant women, nevertheless their role in reducing seroconversion rates is controversial [14-17].
Infection in pregnant women is usually asymptomatic, therefore serological screening can be the only way to detect maternal seroconversion, eventually treat the mother to avoid vertical transmission and reduce fetal damage. On the other hand, diagnosis of congenital infection can be made early and follow-up can be provided.
Without previous knowledge of the pre-conception serologies, it may be difficult to date the infection when anti-Toxoplasma IgG and IgM antibodies are both positive in the first screening. The presence of IgM, which may persist for years, is not absolute evidence of recent toxoplasmosis. Further testing is required to estimate the timing of infection with respect to the date of conception. Anti- Toxoplasma IgG avidity and sequential serological testing should be used to determine the date of maternal infection [18-20]. Prenatal diagnosis should be offered with an amniocentesis to perform a polymerase chain reaction (PCR) for toxoplasmosis DNA in the amniotic fluid (AF). This procedure should not be performed before 18 weeks of gestation nor less than 4 weeks after the estimated date of maternal infection. The parasite can be retained in the placenta for as long as 16 weeks until it is transmitted to the fetus and this can be an important cause of false negative AF test [1]. Fetal blood sampling has gradually been abandoned because it has higher risks for the fetus [21,22].
In the postnatal period, the gold standard to establish a diagnosis of CT is the persistence of anti- toxoplasmosis IgG by 12 months of age [23,24].
Effective anti-toxoplasmosis treatment, if administered early during pregnancy, has been shown to significantly decrease vertical transmission as well as improve clinical outcomes [8]. Current recommended regimens are not effective against chronic bradyzoite stage and do nor eradicate encysted parasites in the tissues [1].
In our hospital, and according to the current standard of care for toxoplasmosis in pregnant women in Portugal, all pregnant women with probable infection diagnosed before 16 weeks of gestational age and infection of the fetus not documented or suspected, should be treated with oral spiramycin. Treatment should include pyrimethamine, sulfadiazine and folinic acid if there is a positive amniocentesis or if seroconversion is detected after 24 weeks of gestational age even without a diagnosis of fetal infection, because the likelihood of the fetus being infected increases with gestational age while the PCR sensitivity in amniotic fluid decreases. Follow-up ultrasound scans are performed every 4-6 weeks until delivery. Amniocentesis is offered at 18 weeks of gestation and 4-6 weeks after presumed infection. The AF is tested for T. gondii DNA using PCR.
Newborn samples are analyzed at the Reference Parasitology and Mycology Laboratory of the National Institute of Health, Dr. Ricardo Jorge. They are tested with real-time PCR for T. gondii DNA, and other serological methods like Immunoglobulin Immunosorbent Agglutination Assay (ISAGA), Enzyme-Link Fluorescent Assay (ELFA) or Immunoassay and Enzyme linked Immunosorbent Assays (ELISA). The infant’s immunological profile is compared to that of their mothers. Mouse inoculation with organic fluids (mothers and newborns blood, placenta, amniotic fluid) is also done.
Newborns are treated with spiramycin or with sulfadiazine, pyrimethamine and folinic acid supplementation, depending on the degree of suspicion of maternal seroconversion and fetal infection (Table 1). Initiating treatment is recommended even in asymptomatic newborns. A negative PCR result in neonatal blood has been used as criteria suspend treatment, but serological follow-up continues until at least 12 months. The follow-up includes a monthly visit for clinical evaluation, laboratory tests for specific anti-Toxoplasma antibodies IgG and IgM every three months, fundoscopic exam and transfontanellar ultrasonography. If CT is confirmed, infected babies are treated for a year [25,26].
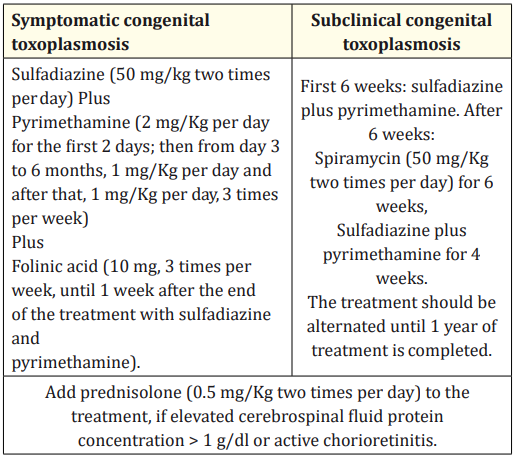
Table 1: Therapy protocol for newborns of the Portuguese Society of Neonatology.
We performed a case-by-case review of the clinical records of children born in a level II hospital between 2008 and 2018, and referenced for suspect CT. Serological, microbiological, clinical and therapeutic data of the mother, fetus and newborn were retrospectively extracted from paper or electronic clinical files available. Data were analyzed using Statistical Package for the Social Sciences ProgramÒ (SPSS Inc., Chicago. IL version 23). The aim of the study was to define the clinical and serological characteristics of children referenced to our hospital with suspected CT in order to optimize diagnostic work-up and treatment.
During the study period, 51 pregnant women were identified as having acquired toxoplasmosis during pregnancy. Median maternal age was 27.8 years (minimum 16 years and maximum 41 years). Almost half of the diagnoses were made in the first trimester (49.0%), versus 19.6% in the second-trimester and 31.4% in the third-trimester.
When it comes to the serological state, 49 women had positive anti-Toxoplasma IgM and 44 women had positive anti-Toxoplasma IgG. In more than half of these (54.5%), the avidity index test was performed: in 10 cases we found IgG with low avidity index, 13 cases presented with high IgG avidity index and one case had intermediate IgG avidity index.
Amniocentesis was performed in only 39.2% of pregnant women and PCR for T. gondii DNA detection in AF was negative in all of them.
Most pregnant women (34 out of 51) received prenatal treatment for toxoplasmosis, 62.7% of them with spiramycin; in two positive anti-Toxoplasma IgM pregnant women there was no data on the medication performed. Of those who did not received treatment, 10 had positive anti-Toxoplasma IgM and IgG in the first trimester, 5 of which without determination of avidity index and 2 of them with low IgG avidity test.
Most of the newborns were male (66.7%) and 96.1% had a term pregnancy. The median weight birth was 3231g (minimum 1730g and maximum 4065g). All children were asymptomatic at birth except for one that presented with chorioretinitis. About 68.6% of infants started treatment immediately after birth: 58.8% with pyrimethamine, sulfadiazine and folinic acid, and 41.2% with spiramycin. Median treatment duration was 2.4 months. All infants had a negative PCR for T. gondii in peripheral blood and no children tested positive for anti- Toxoplasma IgM.
We had four cases of CT, confirmed with a persistent positive antiToxoplasma IgG until after 12 months of age. Clinical characteristics and serologies of the 4 cases are shown in table 2. The estimated incidence in our population was 1.9:10000 (4 cases in 21049 stillbirths in 10 years). One female child presented with chorioretinitis and thalamic calcifications in the transfontanellar ultrasonography at birth (the mother had seroconversion during the 2nd trimester); one male child with no symptoms at birth had with thalamic calcifications in the transfontanellar ultrasonography (seroconversion occurred during the 1st trimester). These two cases were treated for 12 months. Two female children showed anti-Toxoplasma IgG re-elevation around the first year of life (both had maternal seroconversion during the 3rd trimester) and therefore treatment with pyrimethamine, sulfadiazine and folinic acid that had already been discontinued was re-introduced. No neurologic, neurocognitive or neurossensorial impairments were detected.
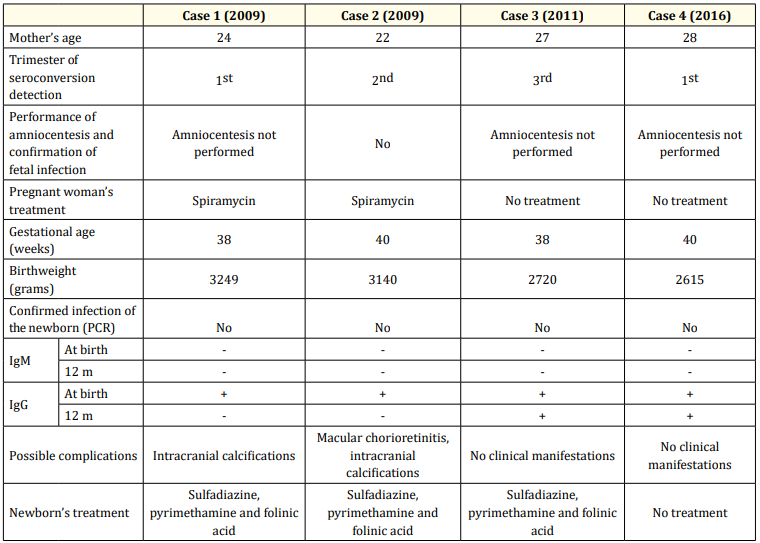
Table 2: Clinical characteristics and serologies of the 4 cases of congenital toxoplasmosis.
There are four groups of individuals in whom the diagnosis of toxoplasmosis is most critical: pregnant women who acquire their infection during gestation, fetuses and newborns who have been congenitally infected, immunocompromised patients, and those with chorioretinitis [4,27].
The first challenge in dealing with CT is the difficulty in proving the infection in the fetus and newborn. Our study confirms the complementarity of prenatal diagnosis and postnatal serological screening for the diagnosis of CT [19]. Regardless of a National Universal Serological Screening for toxoplasmosis in pregnancy in Portugal, our study showed multiple approaches to a positive serological result. IgG avidity was not always determined, amniocentesis was not performed in the majority of cases and one third of pregnant women suspected of toxoplasmosis seroconversion did not received any treatment. In countries with no systematic screening for toxoplasmosis in pregnancy, clinical manifestations were present at birth only in children born to infected mothers, with undiagnosed and untreated CT [28]. In our study, there was only one newborn with clinical manifestations at birth, whose mother-initiated treatment after confirmation of a 2nd trimester seroconversion. In pregnant women with seroconversion to toxoplasmosis, an amniocentesis can be offered, and PCR for T. gondii DNA can be highly sensitivity (around 90%) if used to test AF collected close to the time of the seroconversion and, hopefully, the acute fetal infection [29]. The origin of some false-negative PCR results is still unclear, explanations such as (i) The period between seroconversion and amniotic fluid sampling was too short, (ii) Late transplacental parasite transfer, occurring after amniocentesis has been done (iii) Suboptimal sample transport or storage and (iv) A low fetal parasite burden. Due to low sensitivity of laboratory markers for congenital infection, a negative AF PCR test does not exclude toxoplasmosis and therefore treatment should be maintained irrespectively of the result.
Postnatal diagnosis of CT includes serological, molecular and biological methods but as parasitemia may no longer be present in neonatal blood, especially if the mothers were treated during pregnancy, the sensitivity of PCR detection of T. gondii is reduced. Serological detection of anti-Toxoplasma antibodies becomes the most accurate method of diagnosis [30,31]. The sensitivity of serological test results in newborns is lower in those born to mothers who acquired their infection early in gestation than it is in those born to mothers who acquired their infection late in gestation and/or did not receive treatment [18]. The presence of anti-Toxoplasma IgG at birth reflects placental transfer of maternal IgG antibodies to the fetus and only the follow-up of IgG antibody titters can confirm or exclude CT. The gold standard to establish a diagnosis of CT is the persistence of anti- Toxoplasma IgG by 12 months of age. Conversely, the standard to rule out the diagnosis is the decrease of anti- Toxoplasma IgG titers until its disappearance at£ 12 months of age in the absence of treatment [5].
The second challenge about CT approach is postnatal treatment. In our study all newborns had negative PCR for toxoplasmosis and so treatment was suspended except for two infants with thalamic calcifications in the transfontanellar ultrasonography and one of them with chorioretinitis at birth. They both completed 12 months treatment despite negative PCR detection of T. gondii DNA in neonatal blood and negative serological methods. The other two children with CT stopped treatment after a negative PCR for T. gondii and did an additional course of antibiotics after an increase of antiToxoplasma IgG titters. Our decision to re-treat was based on the management of asymptomatic children proposed by some experts in France [1] that choose to treat only if follow-up serological testing indicates congenital infection, even though it is likely that in this stage of the disease the infant has only cysts, and there is no evidence that the antibiotics used have any effect on the encysted bradyzoite form.
In some congenitally infected infants, mostly from late pregnancy infections, treatment with a pyrimethamine- sulfadiazine combination can result in disappearance of anti-Toxoplasma IgG antibodies during follow-up, leading to a false negative diagnosis. The discontinuation of treatment is followed by a rebound of anti- Toxoplasma IgG titters [5]. This emphasizes the need to maintain serological surveillance in all suspected CT children until at least 12 months of age. If anti-Toxoplasma IgG levels remain negative, assuming the infant is capable of producing IgG antibodies, the diagnosis of CT is excluded.
While some countries in Europe and North America argue in favor of the ethics and cost effectiveness of marginal benefits of screening programs, CT remains an important public health problem that prenatal or neonatal screening or both, may help to improve.
Screening and treatment for toxoplasmosis during gestation result in a decrease of vertical transmission and clinical sequelae.
Effective anti-Toxoplasma treatment, when instituted as early as in utero during has been shown to significantly decrease mother to child transmission and to improve clinical outcomes.
It is imperative that laboratory tests employed for the diagnosis of CT be sensitive, specific, and exhibit high predictive values in order to promptly identify fetuses and newborns that are likely to significantly benefit from treatment interventions.
This work has not received any contribution, grant or scholarship.
The authors have no conflicts of interest to declare.
Copyright: © 2022 Liliana Sá., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.