Gurkan Atay* and Seher Erdoğan
Department of Pediatric Critical Care, Health Science University, Umraniye Research and Training Hospital, Istanbul,Turkey
*Corresponding Author: Gurkan Atay, Department of Pediatric Critical Care, Health Science University, Umraniye Research and Training Hospital, Istanbul,Turkey.
Received: May 03, 2021; Published: January 13, 2022
Citation: Gurkan Atay and Seher Erdoğan. “Serum Albumin Level in Critically Ill Pediatric Patients”. Acta Scientific Paediatrics 5.2 (2022): 03-07.
Objective: Hypoalbuminemia is a frequent condition among patients admitted to pediatric intensive care unit (PICU). The aim of the present study was to determine the prevalence of hypoalbuminemia among critically ill pediatric patients, and to assess its relationship with prognosis.
Materials and Methods: Age, sex, admission diagnosis, Pediatric Risk of Mortality (PRISM) scores, serum total protein and albumin levels, mechanical ventilation (mv) need, number of days on mv, number of PICU days, number of hospital days, and prognosis were recorded among patients admitted to PICU between May 2017 and May 2018. The patients were grouped into two groups on the basis of their serum albumin level; those with hypoalbuminemia were assigned to Group 1 and those without to Group 2.
Results: The study enrolled a total of 126 pediatric patients of whom 64 (50.8%) were female and 62 (49.2%) were male. The mean age of the study population was 64.66 ± 71.28 months. One hundred and five (83.3%) patients survived and 21 (16.7%) died. Fortysix (36.5%) patients had hypoalbuminemia. In patients assigned to Group 1 the need for mechanical ventilation was significantly greater (p:0.007), but there was no significant difference between the number of mv days (p:0.64). Group 1 had a significantly greater PRISM score, a significantly longer hospital stay, and a significantly greater mortality rate (p:0.000, p:0.013, and p:0.000, respectively). No significant difference was seen for the number of PICU days. A prognosis analysis revealed that the survivor group had a higher mean age, less mv need, shorter hospitalization and mv time, and lower serum total protein and albumin levels.
Conclusion: We suggest that serum albumin level is an important prognostic marker as it is a simple, specific, and low-cost parameter that is routinely used in most PICUs.
Keywords: Hypoalbuminemia; Critically Ill Pediatric Patient; Pediatric Intensive Care; Mortality.
Albumin has a long half-life (15-19 days). Among critically ill patients, however, serum albumin level may rapidly drop within 3-5 days. Albumin possesses many physiological roles including regulation of colloid osmotic pressure; binding and transport of various substances in blood, such as drugs and hormones; antioxidant properties; and nitric oxide modulation [1]. Inflammatory process increases albumin catabolism and may reduce its production. During critical illness, capillary permeability is dramatically reduced, leading to albumin exchange between the intravascular and extravascular compartments [2-4]. Previous studies have shown a prevalence of 21-76% for hypoalbuminemia among critically ill children [5-7]. The aim of the present study was to determine the prevalence of hypoalbuminemia and its relationship with prognosis among children admitted to pediatric intensive care unit.
This study was approved by the local ethics committee of Ümraniye Training and Research Hospital. It was conducted retrospectively at a 10-bed pediatric intensive care unit (PICU). Age, sex, admission diagnosis, Pediatric Risk of Mortality (PRISM) scores, serum total protein and albumin levels, mechanical ventilation (mv) need, number of days on mv, number of PICU days, number of hospital days, and prognosis were recorded among patients admitted to PICU between May 2017 and May 2018.
Patients with nephrotic syndrome, liver cirrhosis, protein energy malnutrition, burns, recent cardiovascular surgery, and various disorders that might potentially affect serum albumin level were excluded in addition to those who received parenteral nutrition and albumin infusion prior to admission. Hypoalbuminemia was defined as a serum albumin level of ≤ 2.5 gr/dL among patients younger than 7 months and ≤ 3.4 g/dL among older ones. The patients were assigned to two separate groups as Group 1 and Group 2, which indicate hypoalbuminemia and normal serum albumin levels, respectively. Written informed consent wasn’t obtained from patients who participated in this study since our study is a retrospective study and the data were obtained by screening of the patient files.
The study data were analyzed using IBM SPSS Statistics 22 (IBM SPSS, Turkey) software package. The normality of data distribution was tested with Shapiro Wilk test; the descriptive statistics included mean, standard deviation, frequency. Student’s t test was used for comparison of normally distributed quantitative data and Mann Whitney-U test for non-normally distributed ones. Qualitative data were compared with Continuity (Yates) correction. The statistical significance was set at p < 0.05.
During the study period 154 patients were admitted; twentyeight patients not meeting the inclusion criteria were excluded. There were 64 (50.8%) females and 62 (49.2%) males, making a total of 126 pediatric patients. The mean age was 64.66 ± 71.28 months. The mean serum total protein level was 5.84 ± 1.11(min.3.4-max.8), albumin level: 3.63 ± 0.59(min.2.1-max.4.9). Fifty-three (42.1%) patients received respiratory support via mechanical ventilation. The mean PRISM score was 11.8 ± 10.3; the mean number of mv days was 25.6 ± 30.9; the mean duration of PICU stay was 17.6 ± 24.0 days; and the mean duration of hospital stay was 23,5 ± 25,9 days. One hundred and five 105 (83.3%) patients survived; 21 (16.7%) patients died. Forty-six (36.5%) patients had hypoalbuminemia (Table 1).
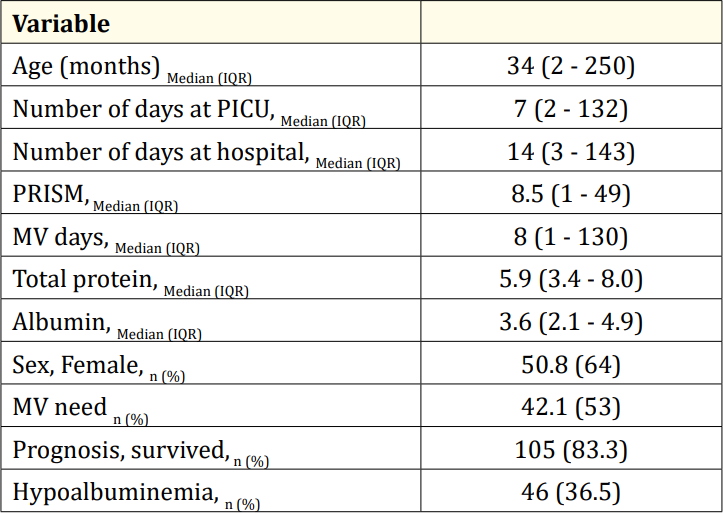
Table 1: Demographic properties of the study population.
PICU: Pediatric Intensive Care Unit; PRISM: Pediatric Risk of Mortality; MV: Mechanical Ventilation.
The female patients had a higher rate of hypoalbuminemia (p:0.03); the mean age of patients in Group 1 was smaller but that difference did not reach statistical significance. Among patients in Group 1, the need for mechanical ventilation was significantly greater although the numbers of mv days were similar. The patients in Group 1 had a higher PRISM score, a longer hospital stay, and a higher mortality rate (p:0.000, p:0.013, and p:0.000, respectively).The number of days spent in PICU were similar (Table 2).

Table 2: Comparison of Group 1 and Group 2.
*Mann Whitney-U Test; + Continuity (Yates) Correction; PICU: Pediatric Intensive Care Unit; PRISM: Pediatric Risk of Mortality; MV:Mechanical Ventilation.
As for the prognosis, the surviving patients had a higher mean age, a less mv need, shorter PICU stay and mv use, and higher serum total protein and albumin levels (Table 3).

Table 3: Comparison of study parameters by prognosis.
*Mann Whitney-U Test; + Continuity (Yates) Correction.
PICU: Pediatric Intensive Care Unit; PRISM: Pediatric Risk of Mortality; MV: Mechanical Ventilation.
Our study showed a hypoalbuminemia prevalence of 36% at admission among patients admitted to PICU. Like previous studies, patients with hypoalbuminemia had a greater mv need, a higher PRISM score, a longer hospital stay, and a higher mortality rate than those with a normal albumin level [7-10].
In a study dated 2016 involving 202 critically ill children, the prevalence of hypoalbuminemia was found 57.9%. Those patients had longer PICU stay and mv use as well as a 4 times higher mortality rate compared to children with normal albumin levels. Likewise, our study demonstrated a greater mortality among patients with hypoalbuminemia; although there was a greater mv need, duration of mv use was not significantly different between the study groups, but the duration of hospital stay was prolonged. Patients in that group were younger but the age difference did not reach statistical significance [8].
Durward., et al. [9], in a similar study, reported a hypoalbuminemia prevalence of 57% at admission, which rose to 76% after 24 hours. They advocated that that increase may have been due to a decrease in albumin synthesis capacity, and a very low rate of use of albumin as volume expander in their unit. However, there was no correlation between mortality and hypoalbuminemia neither at admission nor at 24th hour but they reported that PICU stay was longer in patients with hypoalbuminemia (4.9 vs 3.6 days). They suggested that patients with extreme hypoalbuminemia constituting a minority resulted in that finding.
Tiwari., et al. [10] reported that 21% of 435 critically ill children had hypoalbuminemia at admission, and that rate rose to 34% by the end of the first week and 37% during whole PICU follow-up. That patients had a higher PRISM score (12.9 vs 7.5), a longer PICU stay (13.8 vs 6.7 days), a greater mv need, a longer mv use (p < 0.001), and a higher mortality rate (87.8% vs 16.2%). This effect of albumin on mortality does not solely depend on its regulatory effect on colloid osmotic pressure and capillary permeability, but also on binding lipids and drugs as well as providing a means for transport of trace elements such as copper and zinc in the circulation [11].
The indications for albumin treatment include hypovolemia, shock, burns, hypoalbuminemia, surgery and trauma, acute respiratory distress syndrome, plasmapheresis, and hemodialysis. In critically ill patients who may have endothelial injury, treatment with colloids and crystalloids may increase interstitial fluid volume [12,13]. In the SAFE (Saline vs Albumin Fluid Evaluation) study [14] comprising 6997 patients, 4% albumin or saline was administered when there was a need for fluid need. That study demonstrated no significant difference between mortality rates of both groups. A subgroup analysis revealed that it was beneficial in severe sepsis but detrimental in traumatic brain injury. The EARSS study [15], a randomized controlled, multicenter study, compared patients with early severe sepsis that were administered normal saline and 100 ml 20% albumin, and found no significant difference between both groups with respect to mortality. The ALBIOS study dated 2015 [16] compared patient groups administered crystalloids alone and crystalloids plus 20% albumin and found no significant mortality difference.
A metanalysis comprising 90 studies involving adult patients revealed that every 1 g/dL decrease in serum albumin leads to a 137% increase in mortality, as well as a 28% and 71% increase in the durations of ICU and hospital stay, respectively [17].
The limitations of the present study include having a single-center and retrospective design and involving a heterogeneous patient population.
Serum albumin level is a simple, sensitive, specific, and low-cost marker used in most PICUs; we therefore suggest that it has an important prognostic value in this setting.
(In case of Funding) Funding: The authors declared that this study has received no financial support.
No conflict of interest was declared by the authors.
This study was performed with the permission of Clinical Research Ethical Committee. Since it was a retrospective case-control study, no informed consent was taken.
This article does not contain any studies with animals performed by any of the authors.
Informed consent was obtained from all individual participants included in the study.
The authors declared that this study has received no financial support.
Copyright: © 2022 Gurkan Atay and Seher Erdoğan. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.