Jitendar K Sharma1, Umesh Gupta2, Greeshma Gopalan3, Keerthan RM3 and Kavita Kachroo4*
1 MD & CEO/ ED, Kalam Institute of Health Technology, Andhra Pradesh Med Tech Zone, Visakhapatnam, India
2 Professor, Head of Mats School of Business Studies, MATS Institute of Management and Entrepreneurship, Bangalore, India
3 Scientist -A, Kalam Institute of Health Technology, Andhra Pradesh Med Tech Zone, Visakhapatnam, India
4 Scientist -D, Kalam Institute of Health Technology, Andhra Pradesh Med Tech Zone, Visakhapatnam, India
*Corresponding Author: Kavita Kachroo, Scientist -D, Kalam Institute of Health Technology, Andhra Pradesh Med Tech Zone, Visakhapatnam, India.
Received: November 22, 2021; Published: December 29, 2021
Citation: Kavita Kachroo., et al. “Economic Analysis of Covid-19 Diagnostic Kits in India”. Acta Scientific Paediatrics 5.1 (2022): 02-08.
Introduction: Ever since the first case was reported in December, in China, Coronavirus disease 2019 (COVID-19) has spread over 218 countries and the casualty prediction of the third wave in India. Making it an absolute necessity for a rapid a nd accurate detection and diagnosis of the infection for prevention and containment of the disease. Molecular testing (RT-PCR) is considered to be the gold standard for COVID-19 diagnosis is considered accurate.
Methods: The primary COVID-19 diagnosis is done by Polymerase Chain Reaction (PCR) testing. The most common method used for identifying the genetic material from the SARS-CoV-2 is the real-time polymerase chain reaction RT-PCR. A decision analytical model was prepared for RT-PCR testing for COVID-19 diagnosis by selecting three Made in India diagnostic kits (RT-PCR-X, Y and Z) using Tree Age Pro software.
Results: From the economic evaluation, NMB (Net Monitory Benefit) for RT-PCR Y being ₹ 45,43,740 higher and is the most costeffective intervention as it saves more money compared to other interventions per DALY averted.
Conclusion: Incremental cost-effectiveness ratio (ICER) was calculated, and it is identified that RT-PCR Y is cost effective at ₹ 850 per DALY averted.
PROSPERO ID: PROSPERO CRD42021237539.
Keywords: Cost-effectiveness; COVID-19; Real-time Polymerase Chain Reaction; RT-PCR Kits.
The COVID-19 outbreak has had a huge impact on microbiology laboratories over the past several months. There around 2536 laboratories authorized for COVID-19 testing in India alone. It has brought out the importance of advancements in diagnostics to meet the global demand. The diagnosis of COVID-19 should be based on clinical as well as epidemiological history. As of May 2021, the total tests conducted for the diagnosis of COVID-19 are around 31,30,17,193, with a 7.79% of overall case positivity rate in India [12]. This demand is predicted to rise in the coming days as other countries are facing this, hence there is a necessity of rapid and accurate detection and diagnosis of the infection prevention and containment of the disease [3] (Table 1).
Table 1: Summary of costing methodology of various studies evaluating RT-PCR testing for infectious disease diagnosis.
The COVID-19 tests primarily can be grouped as molecular (NAAT, RT-PCR test), immunoassays, antigen and antibody tests [4]. The primary COVID-19 diagnostic testing is the Polymerase Chain Reaction (PCR) testing [5].
Molecular testing (RT-PCR) is considered to be the gold standard for COVID-19 diagnosis is considered accurate [6]. It is also used to track the spread of disease, identifying strains and mutations but does not detect a prior infection, even one that has recently resolved. Further, a two-step approach using qualitative RT-PCR for detection and quantitative RT-PCR for viral load quantification is recommended for viral load studies. The most common method used for identifying the genetic material from the SARS-CoV-2 is the real-time polymerase chain reaction (RT-PCR) [6]. This involves the reverse transcription of the genetic material (RNA) of the virus to complementary DNA (cDNA). This is then followed by the amplification of some regions of the cDNA.
Cycle threshold or Ct refers to the number of cycles needed to amplify viral RNA to reach a detectable level. The accepted cut off for Ct value globally is around 35-40 and depends on the instructions laid down by different manufacturers [7]. After taking inputs from various virology laboratory experiences, ICMR has now arrived at a single cut off value for Ct that is 35, with a good sigmoidal real-time RT-PCR. All patients with a Ct value < 35 may be considered as positive while those with Ct value > 35 may be considered as negative. All samples with Ct value < 35 with poor sigmoidal curves should be essentially re-tested. Implementing a Ct value cutoff of 24 is not at all advisable as this will lead to missing of several infectious patients and increased disease transmission.
Antigen tests are also used for detecting the SARS-CoV-2 but they are less sensitive than molecular tests and they do not amplify the viral components [6]. A molecular test can be recommended to confirm a negative antigen test result. It detects certain proteins from the surface of the virus and can produce results in minutes. These tests are faster compared to the molecular tests and are less expensive thus more practical to use for testing a larger population [8].
Antibody test also known as serology test, detects IgG and IgM antibodies present and produced by the immune system in response to infection by the virus [3]. It can identify both active and past infections if the antibodies are captured within the timeframe after the onset oft he infection. Accurate antibody testing can identify convalescent plasma donors and identify people who may have immunity. Positive antibody test result indicates that the person most likely would have been infected with SARS-CoV-2 at some point in the past and have may have developed some immunity, though the timing and type of the antibody tests affects the accuracy of the whole test [9] (Figure 1).
Large scale diagnostics are required in containing the spread of COVID-19. Aggressive testingh as helped contain them to a greater extent in countries such as US, Singapore, and Taiwan [10].
Which is the most cost-effective kit among the three kits (RTPCR-X, RT-PCR-Y, RT-PCR-Z) available for COVID-19 diagnosis manufactured in India?
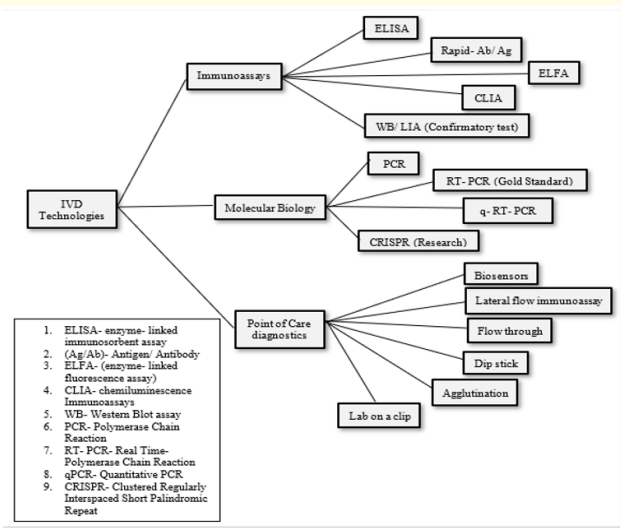
Figure 1: Technologies available for the diagnosis of infectious diseases
In India, bulk testing for COVID-19 is performed majorly using the laboratory method called reverse transcription polymerase chain reaction (RT-PCR). And there is consistently a push to create these Kits locally, given the circumstance, to satisfy the growing needs and to lessen the vulnerability on bringing devices, particularly Diagnostic kits, thereby over all charges can be decreased.
In this study, we compared 3 RT-PCR kits, RT-PCR X, RT-PCR Y and RT-PCR Z in order to understand the threshold for RT-PCR testing for COVID-19 based on the cost, sensitivity, specificity and the outcomes. Here we have considered DALY (Disability Adjusted Life Years) as the outcome measure. The data for cost was collected from primary research. A decision analytical model was built using TreeAge Pro software toi dentify the best RT-PCR kit, to understand the cost difference and to figure out the RT-PCR testing cost for COVID-19.
From our assumption, this economic analysis consists of a total of 1000 susceptible case patients, from which 333 were tested with RT-PCR X, 333 were tested with RT-PCR Y and 334w ere tested with RT-PCR Z. We identified costing studies for RT-PCR kit and outcomes studies for disability weights for diseases and conditions.
Sensitivity and Specificity of the kits were taken into consideration when compared along with the cost and ease of use in order to understand the cost effectiveness threshold for RT-PCR testing. Values of True Positive, True Negative, False Positive and False Negative were calculated using online tool based on these values.
TreeAge Pro is a software used to build and analyze various healthcare models such as decision tree, Markov model, patient simulation model, budget impact model, discrete event simulation and partitioned survival analysis [11]. The models will be built right from scratch, that is developingt he tree structure in case of decision tree, inputting the required parameters, specifying the conditions and running the model and analyzing the outcomes.
The healthcare sector has been facing challenges when it comes to allocation of the available limited resources and costs to meet the demands for services [12]. Advancement in the technology and medical research has led to an increase in the consumed costs. Cost improvements in healthcare can also improve the quality of care available for patients. Cost analysis is one such method that does partial economic evaluation and assesses the cost of various healthcare services provided, programs or interventions. Although cost assessments do not explicitly provide the effectiveness of an intervention/program, their key advantage is reasonably simple analysis of the outcomes (often measured by cost per individual action or unit), ability to directly compare costs between interventions under consideration, with little to no complicated modelling requirement or assumptions that can be having significant influences on the results of an analysis.
Moreover, considering its drawbacks, the effects of cost analysis can be effectively used in decision-making strategies where comparable action evidence on relative efficacy (usually in field demonstration studies) may be cross-examined. The definition of cost analysis differs however based on how one sees it, which can be described in large part in two ways: financialo r economic cost. Financial costs, where costs reflect the cash value of expenses, are also referenced as accounts costs. Economic losses are all costs of decisions or preferences, embodied in the alternate and forgiven advantages.
When the funds are properly budgeted, and fairly spent on areas that need them the most, the efficiency, functionality and quality of the services provided also improves. Quality of the services provided is a growing concern in healthcare as when the cost of technology increases the ability to keep the standards high becomes difficult.
The ultimate emphasis will be on expressing new research laboratory output in addition to established or normal requirements, including adjustments in detection time, workload and adjustments in direct and indirect costs in regards to the currency value, in relation to the number of tests carried out.
In terms of the time-specified units, there are two factors for measuring capital assets. Firstly, the cost of using capital assets of a given process over another laboratory job must be reflected; and secondly, the general differential treatment for potential investment known as time preferences.
Pricing should be averaged for all recurring costs such as raw materials and additives, consumables essential for the requisite diagnostic systems, appropriate human resources and the operating costs. Since many suppliers and distributors sell a product of the same or similar nature at varying rates, and Human resources costs which varies according to years of experience and expertise. The best approach to unify price is to recognize the price of products which use new equipment for testing along with its acquisition cost (Figure 2) (Table 2).
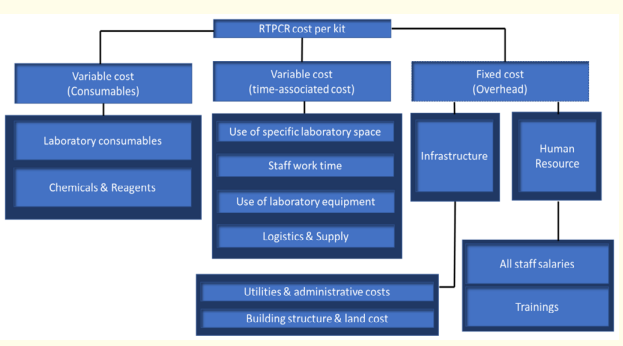
Figure 2: Cost components. RTPCR kit cost consist of variable
cost (consumables), variable cost (time-associated cost) and
fixed cost (over-head).
Laboratory consumables, chemicals and reagents make up the
variable cost. Coming to variable cost based on time, use of
laboratory space, staff work time, use of equipment,
Logistics and supply form the total cost. Logistics is dependent
on the number of kits that has to be transported. Infrastructure
cost like Utilities and administrative costs, building structure,
land cost and Human resource falls under fixed cost. This also
includes rent, electricity, water and maintenance cost. Human
resource includes staff salaries (Scientists, executives,
managers, top management and so on) and training of
employees.
Table 2: Cost components of cost analysis of RT-PCR testing for COVID-19 diagnosis and suggested sources of data.
A decision tree provides the logical structure of decision and possible events as they unfold over time. The decision tree is made up of series of nodes and branches which begins with a decision node (represented by square) with all of the branches off of this node representing the different options available to the decisionmaker, such as treat, no treat or test. Here the options available are the different types of RT-PCR kits. Following this one, we can represent the events that can happen, and as per this economic evaluation, the events considered are True Positive, True Negative, False Positive and False Negative. The probabilities of events or states of nature dictate the chance of going down one branch versus another and can be estimated from data.
This study was aimed to evaluate the cost effectiveness of RTPCR kits for COVID-19 in India. For an economic evaluation to be meaningful, it must attempt to account for all additional costs and health benefits associated with an intervention. In this study, we compared 3 RT-PCR kits (RT-PCR X, RT-PCR Y and RT-PCR Z) in order to understand the threshold for RT-PCR testing for COVID-19 based on the cost, sensitivity, specificity and the outcomes. A decision analytical model was built to identify the best RT-PCR kit, to understand the cost difference and to figure out the RT-PCR testing cost for COVID-19. From the decision analytical model, all the three kits fall under the first quadrant of the cost-effectiveness plane and are cost effective. But, from the Roll-Back analysis it is understood that RT-PCR Y is the best and dominant kit compared to other kits as it saves ₹850.02/DALY Averted, whereas RT-PCR X and RT-PCR Z saves ₹655.53/DALY and ₹619.85/DALY respectively.
Quality of the services provided is a growing concern in healthcare as when the cost of technology increases the ability to keep the standards high becomes difficult. The ultimate emphasis will be on expressing new research laboratory output in addition to established or normal requirements, including adjustments in detection time, workload and adjustments in direct and indirect costs in regards to the currency value, in relation to the number of tests carried out. Our study is an evidence-based analysis which can help policy makers to regulate the cost of COVID-19 diagnostic kits which will eventually lead towards the price rationalization of COVID-19 diagnostic kits. This study can be used a reference for other developing nations as well.
Copyright: © 2022 Kavita Kachroo., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.