Maria Carolina de Campos Martins1, Daniela MLM Ferreira1*, Larissa Prado Maia2, Patrícia Terra Alves2, Aline Teodoro de Paula2, Fernanda M Santiago3, José R Mineo3, Mônica C Sopelete3, Francisco E Martinez4, Morun Bernardino Neto5, Luiz Ricardo Goulart2 and Vânia OS Abdallah1
1Division of Neonatology, Department of Pediatrics, Federal University of Uberlândia, Uberlândia, Minas Gerais, Brazil
2Laboratory of Nanobiotechnology, Institute of Genetics and Biochemistry, Federal University of Uberlândia, Uberlândia, Minas Gerais, Brazil
3Laboratory of Immunology, Biological Science Institute, Federal University of Uberlândia, Uberlândia, Minas Gerais, Brazil
4Division of Neonatology, Department of Pediatrics, University of São Paulo, Ribeirão Preto, São Paulo, Brazil
5Engineering School, University of São Paulo, Lorena, São Paulo, Brazil
*Corresponding Author: Daniela MLM Ferreira, Division of Neonatology, Department of Pediatrics, Federal University of Uberlândia, Uberlândia, Minas Gerais, Brazil.
Received: July 29, 2021; Published: November 26, 2021
Citation: Daniela MLM Ferreira., et al. “Oropharyngeal Colostrum Administration and Anti-inflammatory Effects in Very Low Birth Weight Preterm Neonates”. Acta Scientific Paediatrics 4.12 (2021): 39-47.
Objective: Infections are the main causes of morbidity and mortality in very low birth weight preterm neonates (VLBW PTN) leading to systemic inflammatory imbalance. The oropharyngeal administration of own mother’s milk, particularly colostrum, has been suggested as an immunostimulatory action for protection against neonatal sepsis. As urine has been poorly evaluated in both systemic inflammation and neonatal sepsis, our purpose was to evaluate the effect of oropharyngeal administration of colostrum on the secretion of proinflammatory and anti-inflammatory cytokines in the urine of VLBW PTN.
Methods: From a randomized, double blinded, placebo-controlled trial, urine samples were randomly selected from 55 preterm infants, of which 29 underwent oropharyngeal administration of own mother’s colostrum and 26 received distilled water. Urine samples were collected before and 24 hours after the end of the oropharyngeal administration and analyzed using the Milliplex-27 kit on the MagPix (Luminex) equipment.
Results: A significant reduction in proinflammatory cytokines like IFN - γ (p = 0.005), TNF - α (p = 0.002), IL - 8 (p = 0.012), IL - 9 (p = 0.011), IL -15 (p = 0.012), IL - 17a (p = 0.001) and RANTES (p = 0.018) was observed in the group of children undergoing oropharyngeal administration of colostrum. However, there was no statistically significant difference in the incidence of clinical (OR 1.023; CI 95% 0.344-3.040) and confirmed sepsis (OR 1.158; CI 95% 0.344-3.899) in very low birth weight preterm neonates undergoing oropharyngeal administration of colostrum when compared to the group receiving distilled water.
Conclusion: The oropharyngeal administration of colostrum promoted an anti-inflammatory state, characterized by the reduction of proinflammatory cytokines, which may contribute to the reduction of the incidence of neonatal sepsis.
Keywords: Colostrum; Oropharyngeal Administration; Immune Therapy; Neonatal Sepsis; Premature Infant; Biomarkers
PTN: Preterm Neonate; VLBW: Very Low Birth Weight; NICU: Neonatal Intensive Care Unit; HM: Human Milk; IL - 1β: Interleukin-1 Beta; IL - 1Ra: Interleukin-1 Receptor Antagonist; IL - 2: Interleukin-2; IL - 4: Interleukin-4; IL - 5: Interleukin-5; IL - 6: Interleukin-6; IL - 7: Interleukin-7; IL - 8: Interleukin-8; IL - 9: Interleukin-9; IL - 10: Interleukin-10; IL - 12p70: Interleukin-12p70; IL - 13: Interleukin-13; IL - 15: Interleukin-15; IL - 17a: Interleukin-17a; MIP - 1α: Macrophage Inflammatory Protein-1 Alpha; MIP - 1β: Macrophage Inflammatory Protein-1 Beta; IFNγ: Interferon Gamma; TNF - α: Tumor Necrosis Factor-alpha; RANTES: Regulated on Activation, Normal T Cells Expressed and Secreted; IP - 10: Inducible Protein 10; MCP - 1: Monocyte Chemoattractant Protein - 1
Oropharyngeal administration of colostrum promoted an antiinflammatory state, characterized by the reduction of proinflammatory cytokines, which may contribute to the reduction of the incidence of neonatal sepsis.
Neonatal sepsis is the leading cause of morbidity and mortality in the neonatal period and accounts for about one million deaths in preterm neonates worldwide, particularly those of very low birth weight, weighing less than 1,500 grams [1].
The immune system in VLBW PTN has an immature and ineffective innate response [2]. After bacterial invasion, there is a large increase in proinflammatory cytokines and chemokines that are not restricted to the site of inflammation, leading to dysfunction in other distant organs and tissues, resulting in multiple organ dysfunction [3,4]. Soon after the increase in inflammatory interleukins, the production and release of anti-inflammatory cytokines is initiated and they trigger the body response known as compensatory anti-inflammatory response syndrome (CARS) [5]. The imbalance between the production of proinflammatory cytokines and the anti-inflammatory response is the key point in the rapid clinical decline of the PTN during sepsis and septic shock, as well as the appearance of late sequelae [1,3,5]. Cytokines are by definition the most sensitive and reliable communication method between defense cells modulating the entire immune response, explaining their rapid rise at the onset of innate response followed by a sharp drop in a few hours [3]. Due to these characteristics the choice of a single cytokine for early diagnosis in cases of neonatal sepsis is unlikely [3].
The use of human milk in the VLBW PTN nutrition has been recognized as a protection against the risk of infection during hospitalization [6]. However, clinical instability and gastrointestinal tract immaturity are responsible for the difficulty in early onset and progression of the enteral diet postponing the benefits of this protective effect [7,8].
Therefore, in order to reduce cases of late-onset sepsis and the morbidity and mortality in VLBW PTN, during hospitalization in the NICU, the role of oropharyngeal administration of own mother’s milk, especially colostrum, has been studied as a protective factor against inflammatory damages caused by pathogenic microorganisms [7,12,13]. This hypothesis is based on the presence of high concentrations of bioactive components in colostrum, such as cytokines, which could stimulate the mucosa-associated lymphoid tissue (MALT), present in the oropharyngeal associated lymphoid tissue (OFALT), and in the gastrointestinal tract - (GALT), with lymphocytes migration to distant sites resulting in an immunological activation and amplified anti-inflammatory response [6,8-13].
The role of the kidney in the plasma removal of some proinflammatory cytokines after the diagnosis of sepsis is very important, since it is responsible for the removal of these cytokines from the plasma while diuresis is preserved. The survival of septic patients appears to be inversely related to plasma levels of proinflammatory cytokines. In this context, we hypothesized that inflammatory urinary markers should be reduced in the VLBW PTN under oropharyngeal administration of colostrum if there was a systemic anti-inflammatory action. Therefore, the objective of this study was to analyze the effect of oropharyngeal colostrum administration on urinary excretion of biomarkers panel for neonatal sepsis in VLBW PTN.
This study is part of a randomized, double-blinded, placebocontrolled trial which evaluated the effect of oropharyngeal colostrum administration on the incidence of neonatal sepsis and nutritional progression in 113 PTN with gestational age less than 34 weeks and birth weight lower than 1,500g, born in the Clinics Hospital of the Federal University of Uberlandia, Brazil and admitted at NICU in the same hospital [14]. The study was approved by the Research Ethics Committee of the Federal University of Uberlandia and registered at ClinicalTrials.gov (NCT02912585).
After the analysis of the sample size, 55 VLBW PTN were randomly selected for the present study, of which 29 PTN of the study group receiving colostrum from the mother and 26 PTN of the control group receiving distilled water, according to figure 1.

Figure 1: Flow diagram of patient enrollment. OMC: Own Mother's Colostrum.
In the first 48 hours of the infant’s life, after the randomization and signing the informed consent form by the parents or guardians, all mothers were instructed to manually or electrically pump breast milk (Medela Mini Electric Breast Pump) in the Human Milk Bank, every 2-3 hours, aseptically, under the supervision of a qualified professional. The total amount of breast milk volume pumped was identified and stored in the refrigerator.
For the oropharyngeal administration of colostrum or distilled water, we used the protocol proposed by Rodriguez., et al. [15]. Colostrum or distilled water was fractionated in 0.2 mL sterile syringes (Disposable Syringe 100 UI), properly identified and occluded with opaque tape and referred to the NICU for oropharyngeal administration within the first 48-72 hours of PTN life. The oropharyngeal administration was performed by the nursing technician responsible for the PTN according to the medical prescription in the volume of 0.2 mL every 2 hours for a period of 48 hours. The technique used for the oropharyngeal administration consisted of introducing the syringe containing the own mother’s milk or the distilled water in the right side of the mouth of the newborn and directed to the oropharynx with subsequent injection of 0.1 mL. The syringe was then placed on the left side of the mouth, directed to the oropharynx and the 0.1 mL remaining volume in the syringe was injected. During the procedure, the PTN remained under continuous monitoring of vital signs.
Urine samples were collected prior to initiation of oropharyngeal administration and 24 hours after the end of treatment. The PTN urinary cytokines of both groups were quantified by a multiplex system of the Bio-Plex MagPix (Luminex) analyzer using the Bio-Plex Pro Human Cytokyne 27-plex Assay (Bio-Rad) kit. The following cytokines were analyzed: Eotaxin, interferon gamma (IFN-γ), interleukins (IL) 1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17a, tumor necrosis factor-α (TNF α), inducible protein (IP-10), monocyte chemoattractant protein -1 (MCP-1), macrophage inflammatory protein-1 alfa (MIP-1α) and 1 beta (MIP-1β), and regulated on activation, normal T cells expressed and secreted (RANTES). Data analysis was performed on the equipment’s software and the cytokines concentration was obtained by comparison with the equivalent standard curve of each cytokine to obtain the pg/mL value.
The maternal clinical data and from the entire period of hospitalization of the PTN were obtained through analysis of the medical records. Clinical sepsis was defined as the clinical manifestation of signs suggestive of infection associated with treatment longer than 3 days of antibiotics, and the confirmed sepsis as clinical sepsis with positive blood culture.
All VLBW PTN received HM from the mother or a donor up to the minimum volume 100 mL/kg/day. After this volume, the HM was fortified or the newborn received formula for PTN, depending on the HM availability.
A priori sample size of 39 subjects was calculated to obtain a maximum α of 0.05 and a maximum error of the estimate of 8%.
Statistical analysis was performed using SPSS version 21.0 (SPSS, Chicago, IL, USA) and Bioestat version 5.0 (MamirauáTM, Belém, PA, Brazil) software.
The descriptive analysis included frequencies, percentages and medians.
Odds ratio, Mann-Whitney U test and Fisher’s exact test were used for comparison between groups, and the Wilcoxon rank-sum test was used for comparison within the same group. Statistical significance was defined as p < 0.05.
All VLBW PTN tolerated oropharyngeal administration of colostrum or distilled water with no adverse effects.
The medians of birth weight and gestational age were, respectively, 1114 grams and 28 weeks in the study group and 1107.5 grams and 29 weeks in the control group. The treatment protocol starting occurred with median of 41 hours in the colostrum group and 44 hours in the control group and the median of initiation of the enteral feeding was 3 days in both groups. Other baseline characteristics of VLBW PTN are described in table 1.
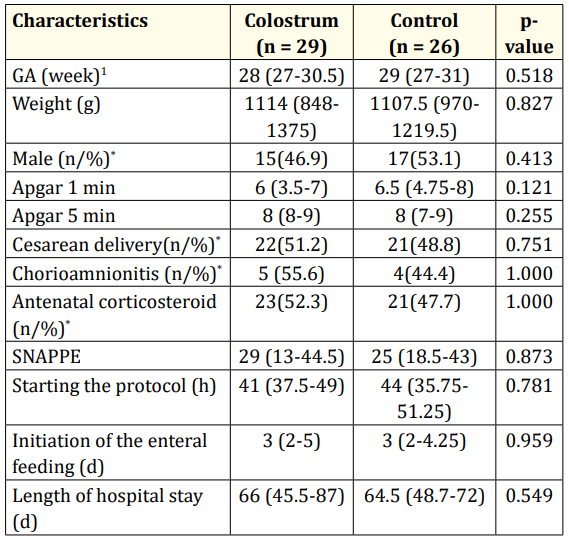
Table 1: Baseline characteristics of the PTN VLBW receiving oropharyngeal administration.
GA (week): Gestational age in weeks; SNAPPE: Score for neonatal acute physiology with perinatal extension; g: Grams; n: Absolute Number; %: Percentage; d: Days; h: Hours.
1Values expressed as median (interquartile range); p-value: MannWhitney U-test for comparison between interval variables; * : Fisher’s exact test for comparison between nominal variables
No statistically significant differences were observed in the occurrence of clinical sepsis and confirmed sepsis in the groups receiving oropharyngeal administration of colostrum or distilled water as shown in table 2.
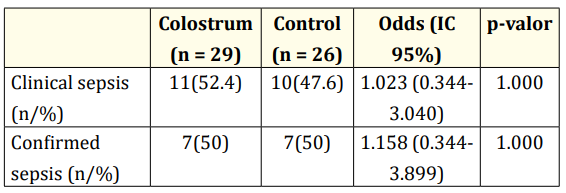
Table 2: Occurrence of sepsis in the PTN VLBW receiving
oropharyngeal administration.
CI: 95% confidence interval; n/%: absolute number/ percentage;
p-value: Fisher’s exact test.
A decrease in the level of several proinflammatory cytokines (TNF-α, IFN-γ, IL-8, IL-9, IL-15, IL-17a and RANTES) was observed 24 hours after oropharyngeal administration of colostrum, which did not occur in the control group. There was no statistically significant difference between both groups before and 24 hours after the oropharyngeal administration. The median of urinary cytokine levels in the evaluated periods are shown in table 3.
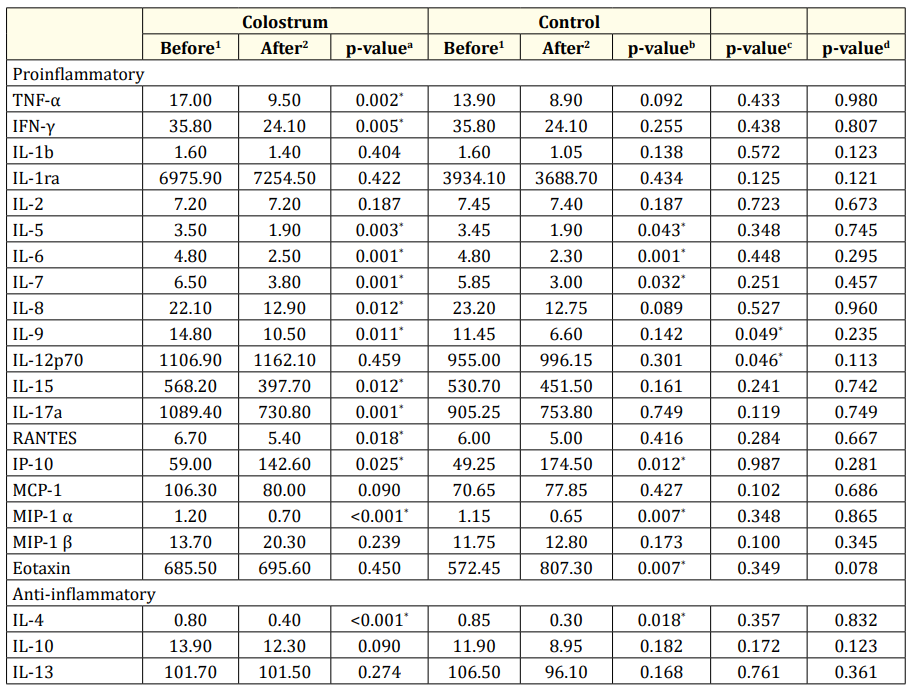
Table 3: Urinary cytokines levels before and 24 hours after of oropharyngeal administration.
1,2: Values expressed in median; p-valuea: Wilcoxon rank-sum test before and 24 hours after oropharyngeal administration of colostrum; pvalueb: Wilcoxon rank-sum test before and 24 hours after oropharyngeal administration of distilled water; p-valuec: Mann-Whitney U-test for comparison between groups before oropharyngeal administration of colostrum and distilled water; p-valued: Mann-Whitney U-test comparing groups after 24 hours of oropharyngeal administration of colostrum and distilled water.
The present study evaluated the urinary level of several neonatal sepsis biomarkers in PTN VLBW submitted to oropharyngeal administration of colostrum and demonstrated a decrease in the urinary level of proinflammatory cytokines (TNF-α, IFN-γ, IL-8, IL-9, IL-15, IL-17a and RANTES). This anti-inflammatory state is probably a consequence of colostrum immune therapy [16-18].
The promotion of an anti-inflammatory and anti-oxidative state has been described as beneficial for the development and growth of the PTN VLBW correlating with the reduction of neonatal sepsis cases [19]. Nevertheless, this study was not able to confirm a reduction in the incidence of clinical and confirmed sepsis in the group that received oropharyngeal administration of colostrum.
Neonatal sepsis is a pathogenic and cytokine-mediated condition that affects immune, anti-inflammatory and blood coagulation homeostasis [2,5]. The course of the disease and clinical symptoms depend on a delicate and complex balance between proinflammatory and anti-inflammatory factors, cytokines with multiple functions and their effects on the immune system [1-3,5,20-23].
Neonatal sepsis is a major challenge in the NICU due to high prevalence and high morbidity and mortality; the use of human milk for PTN VLBW nutrition has been described as responsible for the protective effect against infections [7,13,17,24]. Therefore, it has been considered alternatives methods to human milk administration for PTN due to clinical immaturity and instability that delay the start and progression of enteral nutrition, such as oropharyngeal administration of colostrum [6,8,25].
The present study, although not showing a reduction in the incidence of sepsis in the PTN VLBW submitted to oropharyngeal administration of colostrum, showed a reduction in the urinary excretion of several pro-inflammatory cytokines. These cytokines play an important role in intercellular communication and activation and consequently in neonatal sepsis and have been studied as markers for early diagnosis [16,19,24,26].
Another study conducted by Martin-Alvarez., et al. also proposed that the administration of oropharyngeal mother’s milk contributes to decreasing the pro-inflammatory state of the preterm neonate, indicating a beneficial influence on the inflammatory response as they observed that preterm neonates who received oropharyngeal mother’s milk showed lower serum levels of proinflammatory cytokines (IL-6, IL-8) and higher expression of antiinflammatory cytokines (IL-10 and IL-1ra) [27].
Colostrum contains several bioactive components capable of acting in the lymphoid tissues of the gastrointestinal and oropharyngeal tract transforming the immune response of the PTN VLBW from an inflammatory to an anti-inflammatory state [6,18,21].
TNF-α and IFN-γ are among the cytokines most involved in the Th-1 type innate immune response, primarily proinflammatory response with almost immediate rise after tissue damage caused by pathogens. TNF-α is a cytokine produced by macrophages and the biomarker released faster into the bloodstream, thus being responsible for pyrogen, tissue damage due to necrosis and vasodilation during septic shock. It is related to the severity of infection in the PTN VLBW [28]. In an attempt to counterbalance the inflammatory activity caused by TNF-α, colostrum contains the receptor R1 (TNFR1) responsible for inhibiting the inflammatory and deleterious action of TNF-α, probably, explaining the decrease of this cytokine in the group receiving oropharyngeal administration of colostrum [12]. IFN-γ is also released during the acute phase of inflammation by natural-killer cells with the function of causing cell death [29].
IL-8, one of the chemokines that was decreased following oropharyngeal administration of colostrum, is typically released after bacterial injury and is responsible for attraction and degranulation of neutrophils [28,30,31]. It is considered a potent chemokine and is associated, according to current literature, with the prelude to intestinal mucosa inflammation, particularly in cases of necrotizing enterocolitis [32,33]. In experimental studies, colostrum was responsible for IL-8 suppression in mucosal epithelial cells, mainly in immature cells [11].
RANTES is another chemokine, which decreased significantly in the present study. It is responsible for the attraction of T cells of great toxicity to the site of the injury (T cells - natural killer) besides stimulating the production of IgE and IgG [34]. However, its inflammatory action is still poorly researched during the neonatal period.
IL-9 is an interleukin associated with allergic diseases [35,36]. It is also part of the Th-2 type immune response and may act as a growth factor for T cells and mast cells [36]. There are still few studies in neonates to clarify their real function in cases of sepsis [5,35,36].
IL-17a is part of a newly discovered family (interleukins-17 family with Th17 response) with proinflammatory properties capable of enhancing the TNF-α production [35-37]. It does not yet plays a defined role in cases of sepsis in the neonatal period [19,35,36].
IL-15, a cytokine of the IL-2 family, with cytolytic activity through the activation of natural-killer cells type, is still little explored in the neonatal period as a biomarker for early or late sepsis [35,36].
It is important to note that among the 10 anti-inflammatory urinary markers that significantly decreased in the treated group, 4 (IL5, IL6, IL 7 and MIP-1α) were also decreased in the control group. These findings may be related to an expected evolution of the cytokines levels as it is not known the normal range in urine. The same should explain the increase in the levels of urinary IP-10 in both treated and control groups.
On the other hand, the levels of Eotaxin increased only in control group. Eotaxin is a potent selective chemoattractant for eosinophils, basophils and Th2 lymphocytes which is released by activated endothelial cells, epithelial cells, eosinophils and macrophages. It is readily isolated from allergic tissues, and appears to be a major factor in recruiting eosinophils into tissues. It has been described that the majority of preterm infants develop eosinophilia and abnormalities in eosinophil trafficking during the first period of postnatal life. It could be speculated if the anti-inflammatory state of colostrum had prevented the chemotactic stimuli by reducing eotaxin levels in the treated group.
Currently, there is a great effort to find a reliable biomarker to diagnose neonatal sepsis in PTN admitted at NICU, but there are few studies correlating cytokines and inflammatory status in urinary samples. Urine measurement is quite promising because it is non-invasive and present low-impact on the VLBW PTN care in the NICU [4,38-40]. Furthermore, our hypothesis that inflammatory urinary markers should be reduced in VLBW PTN due to the systemic anti-inflammatory action of colostrum therapy has been successfully demonstrated. The removal of proinflammatory cytokines from plasma was evidenced by high and continuous proinflammatory cytokines in PTN of control group which is supported by an investigation in which the levels of proinflammatory cytokines in the urine of sepsis survivors were inversely related to the levels in plasma.
However, although this study is a pioneer in the use of urinary biomarkers to demonstrate the beneficial effect of oropharyngeal administration of colostrum, it still has limitations due to the small sample size, which should be extended to provide better understanding of its protective function for the VLBW PTN.
We conclude that oropharyngeal administration of colostrum promoted an anti-inflammatory state, characterized by a reduction of the pro-inflammatory cytokines, which may contribute to the reduction of the incidence of neonatal sepsis.
We express our sincere appreciation to the support of Brazilian funding agencies: FAPEMIG, CNPq and CAPES.
Copyright: © 2021 Daniela MLM Ferreira., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.