Sumant Patil1*, Gayatri Bhide2, Rajan Joshi3 and Pratibha Phasale4
1Consultant Paediatric Intensivist, Department of Paediatrics, Deenanath Mangeshkar Hospital and Research Centre, Pune, Maharashtra, India
2Consultant Paediatrician, Department of Paediatrics, Deenanath Mangeshkar Hospital and Research Centre, Pune, Maharashtra, India
3Consultant Paediatrician, Head of the Department of Paediatrics, Deenanath Mangeshkar Hospital and Research Centre, Pune, Maharashtra, India
4DNB Resident, Department of Paediatrics, Deenanath Mangeshkar Hospital and Research Centre, Pune, Maharashtra, India
*Corresponding Author: Sumant Patil, Consultant Paediatric Intensivist, Department of Paediatrics, Deenanath Mangeshkar Hospital and Research Centre, Pune, Maharashtra, India.
Received: September 24, 2021; Published: October 22, 2021
Citation: Sumant Patil., et al. “Clinical Profile of Children with SARS-CoV-2 Infection from a Tertiary Centre in Western India During the First Wave of COVID-19 Pandemic”. Acta Scientific Paediatrics 4.11 (2021): 47-53.
Background: Limited data have emerged describing the presentation and clinical characteristics of the paediatric patients with SARS CoV-2 infection. Majority of this data was from the initial part of the pandemic and mainly from China, some from Europe and USA, although none significant from the Indian sub-continent.
Aim : To describe clinical profile of paediatric SARS CoV-2 infection from a tertiary centre in western India covering the first active duration of COVID -19 pandemic.
Material and Methods: Retrospective observational cohort study over 6 months from 1st April 2020 to 30th September 2020.
Participants
Results: 148 children hospitalised with confirmed SARS CoV-2 infection were included with median age of 8.07 years (IQR 3.07 to 13.11 years) and sex ratio of 1.42 males per female. Of these 57(38.5%) were asymptomatic and 91(61.5%) were symptomatic. Fever was the commonest presenting feature in 65(43.9%), followed by cough 34(23%) and runny nose in 25(16.9%). Pediatric intensive care unit admission was needed in 9(6.1%) patients and there was one neonatal death.
Conclusion: This study confirms that the pattern of disease presentation is mild in pediatric population with a high number of asymptomatic patients and exceedingly rare mortality due to SARS-CoV-2 infection.
Keywords: COVID-19; SARS-CoV-2; RT-PCR; Rapid Antigen Test; Clinical Profile in Children
The year 2020 will go down in the history as the year of the COVID-19 (coronavirus disease 2019). The novel corona virus (severe acute respiratory syndrome coronavirus 2 - SARS CoV2) was found to be responsible for several cases of pneumonia in Wuhan, the capital of Hubei Province in China in December 2019 [1,2]. Very soon the virus showed rapid human to human transmission throughout China and neighbouring countries and later entire world to assume the proportion of pandemic as declared by WHO on 11th March 2020. An intriguing though welcome feature of this infection has been the low rate of mortality and morbidity in the paediatric age group as compared to adult population. However, with increasing testing, a greater number of paediatric cases are being reported with vertical transmission, paediatric patients with adult type manifestations of severe pneumonia, cytokine storms and a very characteristic paediatric inflammatory multisystem syndrome (PIMS) all over the world [3-5]. The trend of clinical presentation in children across the globe has been that of an asymptomatic or mild disease in majority. Few patients requiring hospitalisation or intensive care support mainly those who had underlying childhood morbidities such as chronic respiratory disease, congenital heart disease, children on long term immune suppression and Obesity. Overall, there is minimal mortality seen in children with SARS-CoV-2 infection [6-11]. At the end of the period for data censoring, WHO COVID-19 dashboard (accessed on 7th June 2021) shows India as the second most affected country in the world with 29,439,989 cases behind United States of America [12]. In India, Maharashtra had so far been the worst affected state with a COVID -19 case count at 58,42,000 and Pune remains the city with highest case load at 10,28,957 as per COVID -19 Dashboard of Government of Maharashtra (accessed on 7th June 2021) [13]. Despite the above numbers, there are limited studies available on the pattern of paediatric COVID-19 infections from India during the first wave of pandemic. Hence, we decided to collate the data for clinical profile of paediatric patients from our tertiary centre institute in Pune which encountered a remarkably high case load of patients with SARS CoV2 infection during the first wave of pandemic.
Retrospective observational cohort study conducted over 6 months from 1st April 2020 to 30th September 2020 at a designated tertiary COVID-19 centre, Deenanath Mangeshkar Hospital in Pune, state of Maharashtra, India. During these 6 months a dedicated paediatric fever outpatient department (OPD) was established which assessed children with fever and respiratory symptoms and few those tested positive out of hospital along with their family members. Their nasal and pharyngeal swabs were sent for RT PCR (Reverse transcription polymerase chain reaction) and/or rapid antigen testing as per prevailing hospital policy. In the initial phase of pandemic all patients who were RT PCR positive for COVID-19 were admitted in hospital as per government directives. Home isolation for positive asymptomatic and mildly symptomatic patients was initiated from 1st July 2020 hence those patients were not included for detailed clinical analysis. Ethical clearance was obtained from the local institutional ethics committee before collection of data from hospital records of paediatric fever OPD and online notes of admitted patients in COVID ward. Data from admission to discharge was evaluated throughout the stay in the hospital. Laboratory, radiological investigations and the treatment given was decided by treating paediatrician as per clinical status of the patients.
Descriptive statistical analysis was carried out with the help of SPSS (Version 25) for Windows (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp). The study focussed on analysing pattern of clinical presentation and symptomatology of the paediatric SARS-CoV-2 patients admitted in hospital and their outcome at discharge. The quantitative data has been described in the form of mean with SD (or median and IQR) while in the form of percentages (proportions) for qualitative (categorical) data. The independent t-test was used to determine the difference between two means (a “p” value of < 0.05 was considered significant).
A total of 1721 patients presented to pediatric fever outpatient department of our multispecialty tertiary care hospital during the study period. Of these, 597 (34.6%) patients were positive for SARS-CoV-2; of which 148 (24.8%) were admitted to our hospital and 449 (75.2%) were home isolated from 1st July 2020 onwards (as per the prevailing government policies and guidelines). Figure 1 shows flow of patients seen in fever OPD and subsequent progression. Analysis of the data of 148 hospitalized patients shows a male preponderance with 87 (58.8%) males and 61 (41.2%) females with no remarkable difference in symptomatology. The admitted children were further divided into five subgroups according to their ages (Table 1).

Figure 1: Patient progression flow chart.

Table 1: Distribution of presenting symptoms according to age groups (n = 91).
The median age of the hospitalized children was 8.07 years (IQR 3.07 -13.11 years). The data for the variable “age” was checked for normality. The skewness was 0.016 and the kurtosis was negative 1.3. Thus, the data was found to be normally distributed. The mean age was 8.5 years (SD -5.5 years: 1 day - 17 years, 10 months). The male patients were younger than the female patients (mean age 8.3 years SD 5.5 years and 8.8 years SD 5.4 years respectively), but this difference was not significant (p = 0.578). Overall mean hospital stay of the patients was 6.3 days, SD 3.3 days (range 2 to 32 days). (The median hospital stay was 6 days, IQR 5 to 7 days). Of the admitted patients 109 (73.6%) patients were confirmed positive based on outside reports, while 38 (25.7%) got tested and confirmed in-house. One patient got a positive report from outside and groups (n = 91).
was confirmed by the in-house laboratory, as well. Upon detailed history-taking for contact tracing, positive contacts could be traced in 124 (83.8%) patients; of which 46 (31.1%) patients had either of the parents positive, and 38 (25.7%) patients’ other relatives were positive for SARS-CoV-2. While in 40 (27%) patients, both parents and relatives were positive. For 24 (16.2%) patients contact history could not be traced.
At admission, 57 (38.5%) patients were asymptomatic of which 35 (61.4%) were males and 22 (38.6%) females. Majority asymptomatic patients [17 (29.8%)] were in the age group 10.01 to 15 years, 13 (22.8%) were from the age groups 1.01 to 5 years, 5.01 to 10 years and > 15 years, each. Only one patient was less than 1 year old. Except one, all were cared for in the isolation ward. The mean hospital stay of asymptomatic patients was 5.9, SD 1.7 days (range 3 to 11 days). The symptomatic patients were 91(61.5%) and the most common presenting symptom was fever, which was seen in 65 (43.9%) patients, followed by cough in 34 (23%) patients and runny nose in 25 (16.9%) patients (Figure 2). A combination of fever and cough was seen in 26 (17.6%) patients, while a combination of fever, cough and runny nose was seen in 11 (7.4%) patients. Only 4 (2.7%) patients presented with breathlessness. Table 1 depicts distribution of presenting symptoms when analyzed according to age groups. Overall mean saturation at admission was 97.7%, SD 3.5% (minimum 62% and maximum 100%). Table 2 displays the different ranges of the available laboratory parameters. Absolute lymphocyte count was checked in 122 patients with a mean of 3284/mm³ (range 320-12570/mm³) and platelet counts checked in 122 patients showed a mean of 300000/mm³ (range 11000-593000/mm³). The CRP (C Reactive Protein) value was available in 115 patients and was found to be positive (> 10 mg/lit) in 18 (15.7%) patients. Along with the standard supportive treatment which included paracetamol and antibiotics, HCQ (Hydroxy chloroquine) was used in 10.8% and LMWH (Low molecular weight heparin) was used in 7.4% patients.

Figure 2: Symptoms at presentation (n = 148).
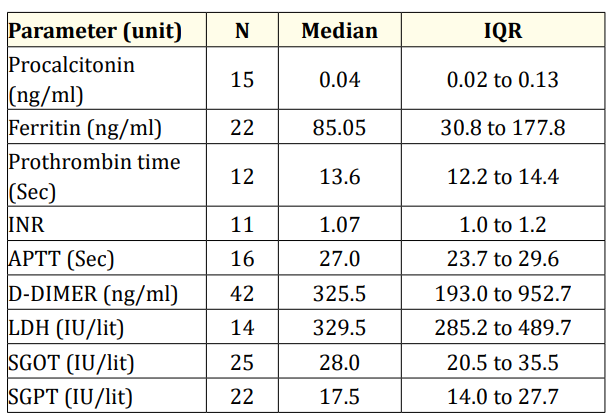
Table 2: Median and IQR values for laboratory parameters.
Of the total 148 hospitalized patients, majority (139; 93.9%) patients were managed in the isolation ward while only 9 (6.1%) required admission to PICU (Pediatric Intensive Care Unit). Oxygen support was required in five patients and HFNC in two patients with a mean SpO2 of 89.8% ± 11.7% (62% to 98%). Of the 9 admissions to the PICU, 5 (55.6%) were male patients. The mean age of patients treated in the intensive care unit was 4.4 years, SD 4.1 years (1 day to 10 years) with a mean hospital stay of 9.3 days ± 8.7 days (3 to 32 days) figure 3. There was one neonatal death.

Figure 3: Comparative clinical profile of two different time zones as per admission strategies of government.
As the pandemic began to evolve over the whole year of 2020, there were gradual increments in the paediatric cases all over globe. We at a tertiary paediatric COVID-19 referral centre started seeing a rapid rise in SARS-CoV-2 infected patients from May 2020 onwards.
We therefore admitted overall 148 SARS-CoV2 infected paediatric patients in April-September 2020 and home isolated 449 patients from July 2020 onwards. During these 6 months a dedicated paediatric fever outpatient department (OPD) assessed a total of 1721 children. Of the fever OPD, 554 paediatric patients were suspected to have SARS-CoV-2 infection, out of those 114 patients turned positive for SARS-CoV-2 infection giving a positivity of 20.57% which outlines a higher disease burden during the pandemic. July 2020 had maximum positivity rate of 34.88% demonstrating the highest infectivity representing the peak of the pandemic in India. Most of the adult studies have demonstrated positivity rates varying between 1% in countries like Australia, South Korea and Uruguay while others had positivity rates between 2050% or even more (Mexico, Bolivia) [14]. The paediatric team attended total 41 deliveries of SARS-CoV-2 positive mothers out of which 1 newborn tested positive for trans placentally acquired SARS CoV-2 infection.
Of the patients admitted with SARS CoV2 infection, majority were in the age group between 5 to 10 years (29.7%) followed by 10 to 15 years (22.3%) while < 1 year was the least common age group projecting a predilection towards toddlers and adolescents. (Table 1). There was a similar pattern of age group distribution suggesting predominance of age group between 1- 15 years in one of the largest paediatric COVID 19 study from the United States [6]. A recent CDC Morbidity and Mortality Weekly Report published January 22,2021on epidemiological trends in children and young population (0-24 years) in the United States showed children and adolescents aged 14-17 years accounted for 16.3%, 11-13 years for 7.9%, those 5-10 years for 10.9% and those 0-4 years for 7.4% [15]. In an interesting comparison of age group distribution with other countries like the United Kingdom, Europe, USA and initial studies from China showed more than double infant population (< 1 year age group) affected than our study [6,7,16-18]. Majority of our patient population had contact history with either parents (31.1%) or relatives (25.7%) or both (27%) who tested positive for SARS- CoV-2 infection depicting a clear-cut evidence of children acquiring COVID-19 infection from household contacts.
In the initial phase of COVID-19 pandemic, as per government of India guidelines all patients were admitted along with their parents and relatives as clusters irrespective of their symptomatology although as the pandemic progressed, we were able to send many patients on home isolation from July 2020 onwards who were asymptomatic or with mild symptoms. There appears a sharp rise in children requiring PICU admission between the two time zones of pandemic from April-June 2020 and July -September 2020 (Figure 3). Overall 38.5% of our patient population was asymptomatic. In one of the largest Chinese study asymptomatic patients were 12.9% [17] while another series had 19.3% of patients asymptomatic [5]. The significantly high number of asymptomatic patients from our patient cohort was in keeping with the pattern from documented adult COVID-19 literature from India [19-21]. Fever was the most common presenting feature (43.9%) followed by cough (23%) and runny nose (16.9%) in the symptomatic patients. The preliminary data from all over world had similar trend [5,16,18].
As the symptomatology was further analysed according to age groups (Table 1), we found that fever, cough and runny nose were the most common symptoms across all age groups followed by gastrointestinal symptoms of abdominal pain, diarrhoea and vomiting which was highest in the age group of 5-10 years and 10-15 years (16.3% to 19.1%). Myalgia and headache were predominantly observed in children above 15 years of age group (20%). PICU admission was required for 9 patients (6%) and majority needed only oxygen support with close monitoring except 2 who needed respiratory support in the form of ventilation for a newborn and high flow nasal cannula support for a 7-month-old child. Mortality was noted in a neonate who had underlying prematurity (32 completed weeks), Very Low Birth Weight (1415 grams ) and developed primary pulmonary hypertension secondary to meconium aspiration at birth. The neonatal death was attributable to the clinical course of typical premature newborn with multiple birth related complications rather than COIVD-19 infection. Overall our clinical data demonstrated an uncomplicated disease severity in the paediatric population admitted in hospital with SARS Co-V2 infection in accordance with the global trend. A study from Washington, DC showed similar clinical trends as this study where 25% paediatric patients required hospitalisation of these 20% were critically ill while 80% were noncritically ill [22]. During the study period we treated 9 cases of PIMS- TS (paediatric inflammatory multisystemic syndrome – temporarily associated with SARS-CoV-2), although not included in our study as they were negative for SARS-CoV-2 PCR. All 9 PIMS -TS cases were treated in PICU and discharged home after treatment.
The laboratory parameters were evaluated as per clinical indications, significant lymphopenia (absolute lymphocyte count < 1500/mm3) was observed in only 9 out of 122 ( 7.37%). This finding was not consistent with the consensus that lymphopenia is considered a remarkable feature of SARS and MERS because of apoptosis and viral particle-induced cytoplasmic damage [23]. However, we are still unclear whether COVID-19 is the same. Henry., et al. assumed that lymphopenia may be related to the severity of COVID-19, and children (especially very young children) with an immature immune system and different immune responses have a low possibility of having lymphopenia [24]. Another interesting aspect of our cohort is that we had only one child with mild asthma as comorbidity which might explain the high number of asymptomatic patients and minimal intensive care support needed to those who were admitted in PICU. It has been observed in general that children with co-morbidities have a higher risk of severe COVID-19 (5.1%) including mortality than children without underlying disease (0.2%) [25]. Radiological evaluations were done only on clinical indications and we found only one child with CT Chest findings of ground glass opacities and lung parenchymal fibrotic changes, who was admitted in PICU requiring prolonged HFNC support. Most of the patients in this study received symptomatic treatment except minority who were given Hydroxy chloroquine (10.8%) Aspirin (1.7%), Low Molecular Weight Heparin (7.4%) and Steroids (2%). As the pandemic spread further in India, ICMR (Indian Council of Medical research) and MoHFW (Ministry of Health and Family welfare, Government of India) guidance became clearer and more paediatric patients were sent for home isolation from our centre. At the time of submission of this study India has been witnessing second wave of the pandemic from March 2021 onwards.
Our study cohort represents a city with highest number of COVID-19 cases from the state of Maharashtra which was worst affected among all of India during the pandemic [13]. Although it may not be representative of whole of India because it is a single centre data collection. It is a retrospective data collected from electronic hospital records it may have its own inherent biases. Most of the children in the study were investigated as per clinical needs and cost constraint, leading to limited number of laboratory or radiological tests done which was not uniform for all patients.
We documented a high number of asymptomatic paediatric patients (38.5%) with COVID-19 infection and our study population had only one patient with underlying morbidity. This study adds to the crucial understanding of clinical pattern of paediatric COVID-19 infection from India as there are limited studies so far on this important aspect of the pandemic from south east Asia.
This largest published cohort of children found to be SARS CoV2 positive in India demonstrates the mild disease severity in this population. We demonstrated a large number of asymptomatic children in our cohort, which may have implications in decision making regarding the timing of reopening of schools, prioritisation of paediatric COVID-19 vaccination, estimating risks of further pandemic waves in India and preparedness for the same.
Copyright: © 2021 Sumant Patil., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.