Barreto Julie1*, Ribeiro Hildenia1, Coelho Fabiana1, Lustosa Amalia1 and Moraes Carlos2
1 Pediatric Gastroenterology Department, Hospital Infantil Albert Sabin, Brazil
2 Pediatric Hematology Department, Hospital Infantil Albert Sabin, Brazil
*Corresponding Author: Barreto Julie, Pediatric Gastroenterology Department, Hospital Infantil Albert Sabin, Fortaleza, Ceara, Brazil.
Received: April 21, 2021; Published: September 06, 2021
Citation: Barreto Julie., et al. “Giant Cell Hepatitis with Autoimmune Hemolytic Anemia- Case Report”. Acta Scientific Paediatrics 5.1 (2021): 09-12.
Giant cells hepatitis associated with autoimmune hemolytic anemia is a rare pathology. An autoimmune disease characterized as a serious liver disease associated with hemolytic anemia. It is still little described in the literature, it has a restrict number of case reports and its pathophysiology is unclear. This study presents a report of two cases of patients diagnosed with giant cells hepatitis associated with autoimmune hemolytic anemia in their first year of age. They show different evolutions of the same pathology displaying the possibilities of manifestation regarding the hepatic and pathological involvement. The patients had severe evolutions, without any response to the first therapeutical measures, had a surprising improvement with the use of anti-b-cell monoclonal antibody treatment, rituximab. The cases of liver disease associated with autoimmune hemolytic must undergo a liver biopsy as early as possible in order to reach a definitive diagnosis. The use of rituximab associated with immunoglobulin infusion has been shown to be an effective treatment and may be considered a first-line therapy, along with a short period of corticotherapy.
Keywords: Giant Cell Hepatitis; Anemia; Rituximab; Rare Disease.
GCH-AHA: Giant Cells Hepatitis Associated with Autoimmune Hemolytic Anemia
Giant cells hepatitis associated with autoimmune hemolytic anemia (GCH-AHA) affects children in early childhood and young teenagers, with higher incidence between three months to two years of age, with no description in the neonatal period.
It is a rare and severe liver disease associate with hemolytic anemia [1].
The pathophysiology is not entirely elucidated. Possibly, an immunological process is involved, however, the disease does not meet the criteria for classic autoimmune hepatitis, even if in some cases the presence of positive autoantibodies is detected. Factors that suggest autoimmune etiology are: sporadic association with other autoimmune diseases mediated by autoantibodies, presence of immune-mediated diseases in family members, response to immunosuppressive treatment and relapse in case of suspension of such therapy [2]. At first, it presents a progressive manifestation, with jaundice and anemia, with an aggressive evolution that, generally, leads to liver failure [3]. The initial findings of jaundice and hepatomegaly appear on average at one year of age. Due to the few reported cases, there are not enough data to define the classic evolution of such pathology [4]. The diagnosis is confirmed by a liver biopsy that shows a histological pattern of a significant alteration of the liver parenchyma, with inflammation and portal and periportal fibrosis, in addition to the presence of multinucleated giant hepatocytes (giant cells that arise due to the infusion of injured hepatocytes). Such findings, however, are not specific to this pathology [5]. The histopathology demonstrates CD68 neutrophils and macrophages, as well as the presence of complement components C3a, C5a, C5b-9 markers, and evidence that the complement cascade is activated in this disease and it is directly related to the inflammatory hepatocyte lesion [3]. Liver disease in cases of GCHAHA is totally refractory to conventional treatment of autoimmune hepatitis, demonstrating that the immune mechanism involved differs between these two pathologies [6]. Prednisone has been used as a treatment since the first description of the disease, but with several reported cases of relapse and serious side effects (systemic arterial hypertension, intraocular hypertension, severe obesity and low height) [12]. B cell-mediated immunity appears to be associated, since the inflammatory infiltrate in hepatocytes is rich in macrophages and neutrophils. That’s why, cases of therapeutic success of treatment using B-cell monoclonal antibodies are being increasingly documented, such as rituximab, a monoclonal antibody that binds itself to the B cell CD-20 and blocks autoreactivity [10]. It is recommended that, initially, it should be used in combination with short-acting corticosteroids because its effect is not immediate [1]. The infusion of intra-venous immunoglobulin can be administered effectively and safely in patients with severe GCH-AHA in the moment of the diagnosis.
The immunoglobulin acts at different levels of the immune response, neutralizing antigens, inhibiting the activation and proliferation of T-cells, in addition to inhibiting the complementmediated tissue damage [7]. Liver transplantation should only be considered if the case is refractory to the treatment with all immunosuppressive drugs and evolves to liver failure, however, with several cases of recurrence even after transplantation [5].
A case report of two patients followed at a tertiary hospital in the state of Ceara, at the hematology and hepatology outpatient clinic, diagnosed with Giant Cell Hepatitis with Autoimmune Hemolytic Anemia. The first patient with initial treatment in November 2017 and the second patient with initial treatment in 2020. Both are still being followed up. Data collection was performed, at first, using theoretical scientific resources. Subsequently, the collection of information contained in the patient's medical record was carried out, as well as the results of relevant exams.
Case 1
MJHC, female, presented jaundice from the first day of life, which evolved with worsening and association with severe anemia. Evidence of hepatosplenomegaly and laboratory tests showing hyperbilirubinemia, at the expense of indirect bilirubin, with increased reticulocytes and positive for coombs test. The hematological study showed the presence of autoantibodies, leading to the diagnosis of autoimmune hemolytic anemia. Corticosteroid therapy was initiated. Over the course of a year of follow-up, she required several hospitalizations due to infectious conditions and the need for transfusion due to severe anemia. Patient presented several side effects due to high-dose corticosteroid therapy, such as severe obesity and hypertension. At the time of attempting to wean off the corticosteroid therapy, she evolved with changes in liver enzymes. Liver biopsy showed hepatitis with mild inflammatory activity and the presence of giant cell transformation of hepatocytes, compatible with the hypothesis of giant cell hepatitis (Figure 1).
After the result, Azathioprine was started.
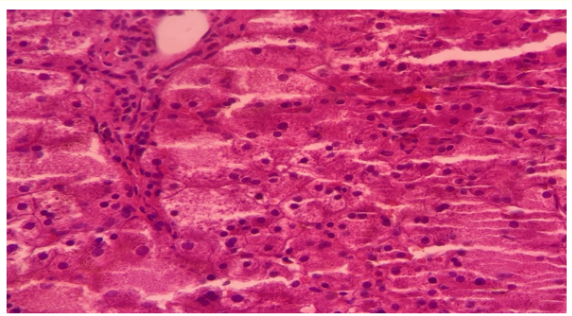
Figure 1: Multinucleated giant hepatocytes with broad cytoplasm.
Source: Pathology file of Hospital Infantil Albert Sabin.
Patient kept being frequently hospitalized, with recurrences and hepatic and hematological changes, even after one year of corticosteroid therapy and six months of using azathioprine, when it was decided to start an empirical therapy with monoclonal antibody, four doses of rituximab were given. Normalization of liver enzymes occurred four months after the fourth dose.
Currently, she hasn’t used medications for two years and also maintained immunological improvement and with normalization of blood counts and transaminase levels. She continues to be monitored.
Case 2
LRO, male, with no neonatal complications or previous comorbidities, started jaundice at one year of age. At first, there was a spontaneous improvement of the condition, the hypothesis of viral hepatitis was considered. However, two months later, he developed jaundice, fecal hypocholia and bloating again. He presented jaundice and hepatosplenomegaly and lab tests with anemia, increased reticulocytes, positive coombs and altered transaminases. Liver biopsy compatible with giant cell hepatitis, large hepatocytes with broad and multinucleated histoplasma. With mild inflammatory changes and perisinusoidal fibrosis. An Immunohistochemistry test was performed for CD20, with the presence of lymphocytes around multinucleated hepatocytes (Figure 2).
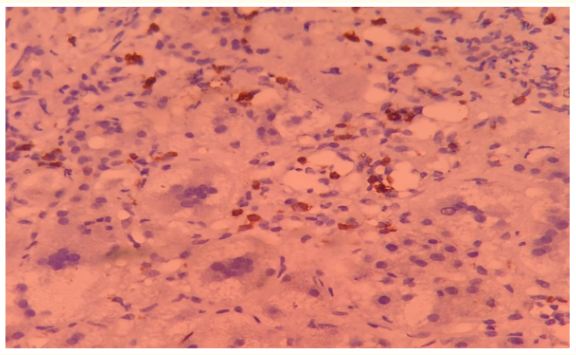
Figure 2: Large hepatocytes with broad histoplasma and multinucleate. With mild inflammatory changes and persinusoidal fibrosis. Arrow: Immunohistochemistry for CD20, with the presence of lymphocytes around multinucleated hepatocytes
Source: Pathology file of Hospital Infantil Albert Sabin.
Corticosteroid and azathioprine therapy was initiated. After four months, the patient continued to show signs of disease activity, with persistent jaundice and choluria, requiring hospitalization due to infectious conditions. Then, the team opted to start the rituximab treatment, with a total of 6 doses of the drug. About a month after the last dose, the anemia was under control, and a decrease of bilirubin and transaminases was noticed. Clinically, there was an improvement in jaundice, irritability and a reduction in waist circumference. The patient continues to be monitored to evaluate the complete clinical response to the drug. The average age described in the literature for onset of GCH-AHA manifestations is around seven months of age [3].
Data that matches the age group which the second patient belonged to when he presented the first symptoms and shows the early onset of the disease in the first.
In a case study carried out in Canada in 2014, four were children followed, and in three the manifestation of hemolytic anemia preceded hepatitis around three to four months, as it happened in the first case, however, it is not yet certain that this is the normal course of the disease, since other reports in which hepatitis was the first manifestation, or which may appear concomitantly, as in the second case [1]. Both patients had several hospitalizations due to infectious conditions. The literature describes that infectious conditions can be considered triggers for recurrences of GCH-AHA, since the infectious agents act as antigens that trigger a cross-reaction, the activation of B cells, and the initiation of the entire cascade of inflammatory reaction [10].
The normalization of liver enzymes occurred approximately four to six months after the fourth dose of rituximab. This is consistent with the fact that B cell restoration in the peripheral blood usually occurs within five to 13 months [1].
The remission of the disease is defined as the normalization of liver enzymes, hemoglobin level and reticulocytes. In five patients in Poland, the first line of treatment with corticosteroids and azathioprine failed and they all presented a complete remission of the disease after using Rituximab. The minimum of four doses is considered ideal, and additional doses may be considered in cases of recurrence or incomplete remission [3].
The big question in the follow-up of patients with GCH-AHA is when to confirm a complete remission of the disease and how long are relapses still possible. According to Annibali., et al. [5], it took 19 months to confirm a complete remission. As for Gómez e Valverde [9], they argue that relapses may occur, even with a controlled liver damage and that the immunosuppressive therapy should continue for about five years.
The cases described here reinforce the importance of knowledge about rare pathologies. The cases of liver diseases associated with autoimmune hemolytic anemia should undergo a liver biopsy as early as possible in order to establish a definitive diagnosis. The use of rituximab combined with the infusion of intravenous immunoglobulin has demonstrated to be an effective treatment, and may be considered a first-line treatment, along with a short period of corticosteroid therapy. The patients in this study are still being monitored so we may record any possible relapses and the need for additional doses. So far, a clinical and laboratory improvement shows a good therapeutic response to the use of rituximab, which may be considered an effective and safe therapy for the remission sustained in this cases.
However, there is still a need for further studies about the topic, as the duration of the induced remission and long-term side effects are still unknown.
I have no conflict of interest to declare
Copyright: © 2022 Barreto Julie., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.