Márcio Luís Duarte1, Thaís Amanda Frank de Almeida Alves2, Marco Antonio Alves Braun2, Lucas Ribeiro dos Santos2 and Élcio Roberto Duarte3*
1Webimagem Telerradiologia, Brazil
2Faculdade de Ciências Médicas de Santos, Brazil
3Irmandade da Santa Casa de Misericórdia de Santos, Brazil
*Corresponding Author: Élcio Roberto Duarte, Department of Ultrasonography, Irmandade da Santa Casa de Misericórdia de Santos, Brazil.
Received: August 13, 2021; Published: August 27, 2021
Citation: Élcio Roberto Duarte., et al. “Patau Syndrome - A Rare Trisomy”. Acta Scientific Paediatrics 4.9 (2021): 02-05.
Patau syndrome is a rare chromosomal syndrome, with a prevalence of 1 case every 5000 - 12000 births, being the third most common multiple malformations. In most cases, the extra chromosome 13 for trisomy comes from the mother. However, in 80% of the cases, a mutation occurs with non-separation in maternal meiosis. It is characterized by multiple and varied malformations, the main abnormalities being severe mental retardation, hypotonia, skeletal and midline malformations, facial defects, holoprosencephaly - being the most common the alobar form, cardiac defects, omphalocele and polydactyly, besides presenting a survival. too short, rarely reaching childhood. At ultrasonography, evaluation of the fetal face (including ears) and extremities (including hands and feet) with the extensive use of fetal echocardiography increases the sensitivity of the method to detect pathology.
Keywords: Fetus; Infant; Newborn; Trisomy 13 Syndrome; Ultrasonography; Prenatal Care
Patau syndrome is a rare chromosomal syndrome, initially described in 1960 by Patau with a prevalence of 1 case every 5000 - 12000 births, being the third most common multiple malformations [1]. It rarely presents mosaicism, occurring in only 5% of patients, with a variable and understandable phenotype [2]. In most cases, the extra chromosome 13 for trisomy comes from the mother [3]. However, in 80% of the cases, a mutation occurs with non-separation in maternal meiosis [4].
It is reported that miscarriage or stillbirth within 12 weeks of pregnancy occurs in 49% of cases, and between 18 weeks and 40 weeks was 42%. From the 24th week, about 35% [5]. Only 33% of fetuses with trisomy 13 can be born alive [6].
It is characterized by multiple and varied malformations, the main abnormalities being severe mental retardation, hypotonia, skeletal and midline malformations, facial defects, holoprosencephaly - being the most common the alobar form, cardiac defects, omphalocele and polydactyly, besides presenting a survival. too short, rarely reaching childhood [7]. The clinical course can also be modified by other associated anomalies such as polyhydramnios, oligohydramnios, intrauterine growth retardation, umbilical cord with single umbilical artery, eyes defects - congenital glaucoma, and polycystic kidneys [8]. Some skin defects have been reported as scalp defects, glabellar spots, deep palm wrinkles, convex soles, and multiple hemangiomas [9].
Its diagnosis is suspected in prenatal exams, the morphological study by ultrasonographic examination detects about 90% of type assessment or by a cordocentesis and/or amniocentesis during pregnancy [9].
Prenatal screening and maternal age have dramatic effects on the prevalence of live births from these pregnancies [10]. Survival in the disease remains virtually unchanged with most dying in the neonatal period [10].
Female patient, 1 day old, caucasian, born by cesarean with the presence of meconium, after 39 weeks of pregnancy, Apgar on the first minute scoring 1 and 7 after 5 minutes, weighing 1600g, 24 cm of cephalic perimeter and 43 cm in length, hypotonic, heart rate of 70 beats per minute and irregular breathing. After emergency procedures, the heart rate rase up to 120 beats per minute. Physical examination showed tachypnea, microcephaly, absence of the nose, extensive cleft palate, hypophonetic heart sounds, bilateral crackles, innocent abdomen without visceromegaly’s, ambiguous genitalia, umbilical cord with 1 artery and 1vein, skin with vernix stained with meconium, and polydactyly in both hands. Readily transferred to the neonatal intensive care unit (ICU).
The mother, 46 years old, having no previous pregnancy or birth complications. Nine held consultations in prenatal, with obstetric history of having minor bleeding in the 1st trimester of pregnancy and abnormal vaginal discharge on the 7th month of pregnancy being treated with Nystatin. Serology for syphilis, HIV, toxoplasmosis, hepatitis B, and C showing negative IgG and IgM. Father with 51 years, without vices. There is no consanguinity between the parents and grandparents, both paternal and maternal.
At the 1st morphological ultrasound, performed at 14 weeks of gestation, it was found a normal nuchal translucency (1.1 mm), but no nasal bone, no characterization of the central nervous system midline anatomy, polydactyly in both hands (Figure 1), reverse wave A of the ductus venous Doppler, placental edge overlying the internal cervical canal and the umbilical cord with a single umbilical artery (Figure 2), indicating research of fetal karyotype and fetal echocardiography.
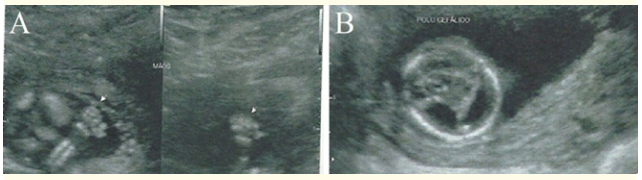
Figure 1: In A, ultrasonography demonstrating polydactyly in the hands. In B, ultrasonography displaying the lack of characterization of the midline anatomy of the central nervous system.
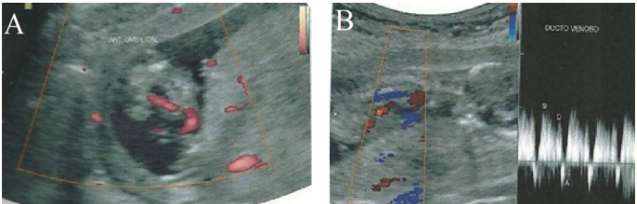
Figure 2: In A, ultrasonography demonstrating an umbilical cord with a single umbilical artery. In B, ultrasonography displaying wave reversed to the Doppler study of the ductus venosus.
The 2nd morphological ultrasound, performed at 21 weeks of gestation, characterized holoprosencephaly, microcephaly (Figure 3), facial dysmorphism (bilateral cleft lip, nose agenesis, and hypotelorism) (Figure 3), polydactyly, single umbilical artery, cardiopathy (ventricular septal defect and left heart chambers hypoplasia), intrauterine growth retardation.
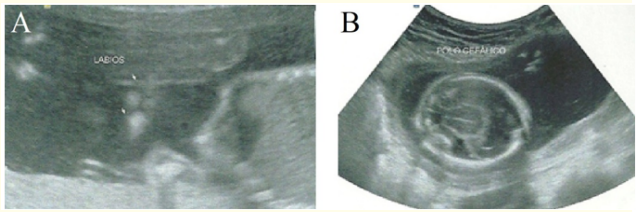
Figure 3: In A, ultrasonography demonstrating facial dysmorphism (bilateral cleft lip). In B, ultrasonography displaying holoprosencephaly and microcephaly in B.
After two weeks of birth, a head computed tomography (CT) scan was performed, which visualized convergent strabismus, ventricular systems dilatation, no characterization of the septum pellucidum - alobar holoprosencephaly - enlarged subarachnoid space, absence of cortical gyri (lissencephaly), overlapping skull bones and blood bordering the cerebellar hemispheres (Figure 4).
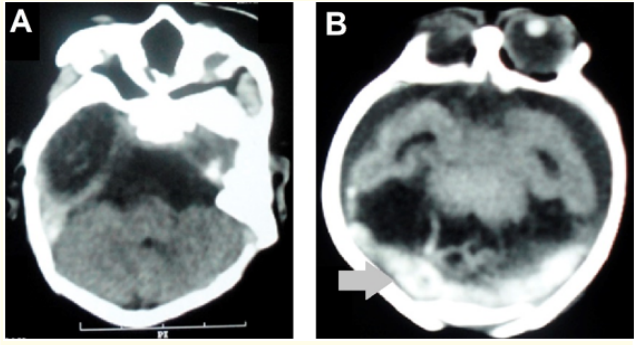
Figure 4: Head CT in the axial section in A and B demonstrating dilation of the systems ventricular without septum pellucid characterization (alobar holoprosencephaly), increase of the subarachnoid space and absence of cortical gyri (lissencephaly) and blood bordering the cerebellar hemispheres (gray arrow).
Karyotype test was done by G banding, confirming the trisomy of 13 chromosomes with the karyotype 47, XX, +13 - Patau Syndrome. After been transferred to the neonatal intensive care unit (ICU), she survived for 1 month until she died of acute respiratory failure.
The main structures analyzed in the ultrasound of the first trimester are the fetal nuchal translucency thickness, fetal heart rate, fetal nasal bone, fetal tricuspid regurgitation, ductus venous flow fetal, crown-rump length, fetal trunk and head volume, fetal front maxillary facial angle, gestational sac volume, and umbilical cord diameter [11].
The positive predictive value of fetal nuchal translucency (cutoff of 2.5 mm) increased as a screening test for the detection of fetus chromosomal abnormalities is 14.8% for genetic malformations in general, with 2.45% for Patau Syndrome, which was confirmed after genetic testing corroborative [12]. However, the most common ultrasonographic changes, regardless of the fetal echocardiography, are holoprosencephaly and facial defects [13].
On examination between 11 and 13 weeks, the increased nuchal translucency measurement and fetal heart rate, associated with assessment for holoprosencephaly and omphalocele can identify 90% of fetuses with 13 trisomies [14]. Between 16 - 22 weeks, all cases presented any changes to the ultrasonographic examination, morphological or not, as the polyhydramnios and intrauterine growth retardation [15].
Cardiac abnormalities are common in Patau Syndrome, with studies in the literature citing it as the most recurrent alteration followed by abnormalities in the central nervous system and facial anomalies, being diagnosed more commonly in the second and third gestation trimesters, in association, in most cases with cystic hygroma, nuchal edema, fetal hydrops, omphalocele, diaphragmatic hernia, limbs abnormalities, such as clubfoot, genito-urinary abnormality, and polydactyly [16]. Fetal measurements, biparietal diameter, abdominal circumference and femur length are below the 5th percentile in the second trimester in about 50% of cases [16].
Unlike Down Syndrome, the measurement of the fetal iliac angle in the second trimester has no value in the diagnosis of trisomy 13 [17]. However, in some cases, a single change should lead to suspicion of trisomy, such as the finding of multiple bilateral papillary muscles [18].
There are reports with 100% sensitivity and 98.9% specificity for diagnosing prenatal genetic noninvasive 13 trisomies by sequencing of maternal DNA [19]. In ultrasound, evaluation of fetal face (including ears) and the extremities (including hands and feet), with extensive use of fetal echocardiography increases the detection method sensitivity of the pathology [20].
The ultrasound exam is a sensitive exam for the suspicion of Patau’s Syndrome, especially when there is holoprosencephaly and facial alterations in the second trimester of pregnancy. However, the definitive diagnosis is the karyotype. The combination of these two methods, according to the medical literature, proves to be faithful to intrauterine diagnosis, performing the genetic analysis through cordocentesis or amniocentesis.
The authors declare that there is no conflict of interest regarding the publication of this paper.
Copyright: © 2021 Élcio Roberto Duarte., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.