Enas Elnagar1*, Amal Abdel-Hameed2, Hanan Omar3, Mohamed El-Kalioby4 and Abdelmoneim Khashana5
1Lecturer of Pediatrics, Faculty of Medicine, Suez Canal University, Egypt
2Faculty of Medicine, Suez Canal University, Egypt
3Associate Professor of Clinical Pathology, Faculty of Medicine, Suez Canal University, Egypt
4Professor of Pediatrics, Faculty of Medicine, Suez Canal University, Egypt
5Associate Professor, Faculty of Medicine, Suez Canal University, Egypt
*Corresponding Author: Enas Elnagar, Lecturer of Pediatrics, Faculty of Medicine, Suez Canal University, Egypt.
Received: May 24, 2021; Published: July 14, 2021
Citation: Enas Elnagar., et al. “Decreased Platelet Parameters and Serum Uric Acid Levels Suggest Possibility of Acute Phase Reaction of Neonatal Sepsis”. Acta Scientific Paediatrics 4.8 (2021): 35-40.
Neonatal sepsis represents an important cause of morbidity and mortality especially in low-resource settings. Early diagnosis and prompt treatment of neonatal sepsis improves outcome. The aim of this study is determine the role of platelet indices and serum uric acid level in the early diagnosis of neonatal sepsis and early detection of neonatal sepsis with easily accessible, inexpensive, and widely used laboratory tests. Retrospective case-control study the target population of this study consisted of 120 neonates who were divided into two groups, cases: 60 neonates who had clinical picture of sepsis with or without positive blood culture, and controls 60 healthy non-septic neonates of the same age and maturity. Platelet parameters followed in complete blood picture (CBC), and serum uric acid of all neonates. Our results showed decrease platelet parameters and serum uric acid in cases of neonatal sepsis so we can use these tools as predictors of neonatal sepsis. There was significant difference of platelet parameters and serum uric acid between cases and controls, significantly lower in neonates with sepsis compared to healthy controls. Decreased platelet parameters including (platelet count, mean platelet volume (MPV), platelet distribution width (PDW) and platelet crit (PCT) values suggest possibility of sepsis and should alert clinicians to the possibility of sepsis and to initiate or change antibiotic treatment. There was statistically significant difference between cases and controls regarding to uric acid.
Keywords: Neonatal Sepsis; Platelet Indices; Serum Uric Acid
Sepsis, which occur in neonatal period a result of the effects of a bacterial infection [1]. Immaturity of the neonatal immune system leads to immunodeficiency, as reflected by the increased susceptibility to infections with both viral and bacterial pathogens [2]. Sepsis is responsible for almost one and a half million deaths every year worldwide [3] and the neonatal infections with impaired cortisol production have a worse prognosis [4,5].
One of the hemogram parameters affected by many inflammatory conditions is the mean platelet volume (MPV) [6], where MPV can predict the development and severity of sepsis in neonates [7].
In vitro, by scavenging free radicals and serum uric acid (SUA) has antioxidant properties, the latter preventing iron-catalyzed oxidation. The concentration of SUA in biological fluids has a clear association with demonstrable antioxidant activity [8] free radicals are involved in pathogenesis of neonatal septicemia [9].
Urate is a potent antioxidant that can scavenge superoxide, hydroxyl radicals and single oxygen at physiological concentrations [10].
A basic biomarker of inflammatory process is the neutrophil -lymphocyte ratio (NLR). In addition, a useful marker of systemic inflammation is the platelet lymphocyte ratio (PLR) [11]. So, this study was carried out to determine the role of platelet indices and serum uric acid level in the early diagnosis of neonatal sepsis to allow rapid and more accurate diagnosis.
This study was carried out as a retrospective case-control study and was conducted in the neonatal ICU of Pediatric department in Suez Canal University Hospital.
The studied patients were divided into two groups: 1- patient group (60 neonate): that include patients diagnosed with neonatal sepsis based on clinical diagnoses. 2- Control group (60 neonate): that include neonates with no symptoms or signs, for infections.
Serum CRP level for neonates in this group was negative.
Preterm and full term neonates from first day to 7 days old. 2- Presence of documented highly possible sepsis criteria according to the following [12]: a) at least three sepsis related clinical signs: apnea, temperature instability, need for supplemental oxygen, bradycardia, tachycardia, hypotension, feeding intolerance, abdominal distension. b) CRP > 6 mg/dl. c) Altered blood parameters in addition to CRP: Total leucocytic count with differential, Platelet count. d) Blood culture: positive or negative.
Exclusion criteria: Neonates with condition which may affect the platelet parameters other than sepsis e.g. Inborn errors of metabolism, Major congenital anomalies and cases of hypoxic ischemic encephalopathy. Sample size was calculated according to the following formula [13]:
Where: n= sample size, Z α/2 = 1.96, Zβ = 0.84, σ = the estimate of the standard deviation (in the study group), µ1 = mean platelet volume in sepsis group = 332.4 [7], µ2 = mean platelet volume in the control group = 310 so, the total sample size is (60).
All neonates were subjected to complete history taking, clinical examination, and laboratory investigations, complete blood picture (CBC) [14] including blood indices, total leucocytic count (TLC) with differential count, neutrophil lymphocyte ratio (NLR), platelet lymphocyte ratio (PLR), platelet count, mean platelet volume (MPV), platelet size distribution width (PDW) and plateletcrit. Creactive protein (CRP) [14]. Estimation will be carried out using the test kit, the AVITEX- CRP latex particles are coated with antibodies to human CRP. When the latex suspension is mixed with serum containing elevated CRP levels on a slide, clear agglutination was seen within two minutes. Specimen collection and storage: Fresh sample of venous blood and allow clot to form and retract centrifuge clotted blood sample and collect clear serum, store at 2-8°C AVITEX-CRP had a detection limit of 6 mg/L of CRP in the patient's serum [15]. Uric acid: determined by enzymatic test, using the Spin react kit, Spain catalog No: 41000 [16].
All statistical analysis was carried out using statistical package for social sciences (SPSS) for windows version 23.0 (SPSS, Chicago, IL, USA). The t-test was used to compare the means of the groups. Comparisons between categorical data were performed using the chi-square test and Mann Whitney test. A P value of <0.05 will be considered statistically significant.
Written consent was obtained from parents of all participants with full explanation of hazards and benefits of the procedure.
The present study included two groups. Group 1 (n = 60): that included patients diagnosed with neonatal sepsis based on clinical diagnoses and Group 2 (n = 60): healthy neonates who served as a normal control group.
Baseline characteristics of the studied sample are described in table 1. Males represented the majority of the participants in both groups (68.3% of the cases and 76.7% of the controls). Moreover, the majority of the participants in both groups aged between 4 to 7 days (96.7% of the cases and 91.7% of the controls).
The majority of the participants in cases were preterm (≤ 37 week) (53.3%), while the majority of the participants in control group had a full-term pregnancy with no statistically significance (p = 0.201).
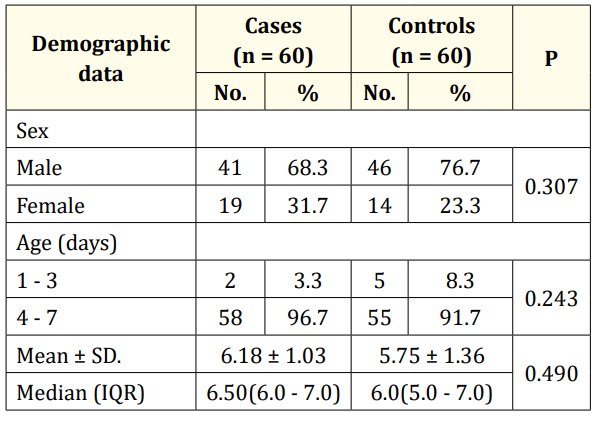
Table 1: Comparison between the two studied groups according to demographic data.
Comparison between the two studied groups according to WBC parameters showed that cases had significantly higher total leucocytic count (15.01 ± 7.78) compared to that of controls (11.63 ± 5.74) (p < 0.05).
Table 2 shows that there is no statistically significant difference between cases and controls in regard to the Platelet-to-lymphocyte ratio (p = 0.737) and Neutrophil-Lymphocyte Ratio (p = 0.803).
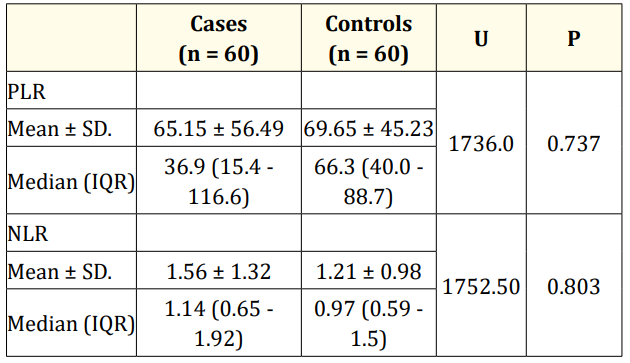
Table 2: Comparison between the two studied groups according
to platelet-lymphocyte ratio (PLR) and neutrophil- lymphocyte
ratio (NLR).
U: Mann Whitney test.
Table 3 shows platelets indices parameters among cases and controls. It shows that cases with neonatal sepsis had significantly lower platelet count (p < 0.05), mean platelet volume (p < 0.05), platelet distribution width (p < 0.05) and plateletcrit (p < 0.05) than control group.

Table 3: Comparison between the two studied groups according to platelet indices parameters.
The three most common organisms found among the cultures were staphylococcus aureus (36.7%), followed by Klebsiella (15%) and E. coli (13.3%).
The relation between platelet indices, uric acid and CRP of cases with deferent blood culture results. It shows that patients with staph. hominis and pseudomonas aeruginosa had the highest CRP values (92 ± 58.99 mg/L and 50.20 ± 34.25 mg/L, respectively). Moreover, patients with staph. hominis had the lowest platelets count (166 ± 134.13 x 103/cmm) while patients with pseudomonas aeruginosa had the highest MPV (11.70 ± 1.69 fl). Regarding serum uric acid, patients with E. coli had the highest serum uric acid levels (5.46 ± 2.26 mg/dl) while patients with Chlamydia had the lowest serum uric acid levels (3.93 ± 0.81 mg/dl).
Table 4 shows that there is no statistically significant correlation between CRP, platelets indices parameters and uric acid among cases. Moreover, there is statistically positive significant correlation between gestational ages with uric acid among cases.
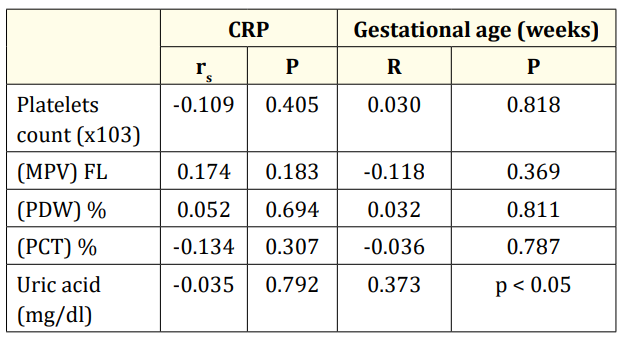
Table 4: Correlation between CRP, gestational age (weeks) and different parameters in cases group (n= 60). r: Pearson coefficient; rs: Spearman coefficient.
Patients with neonatal sepsis had significantly lower mean uric acid level than healthy controls (4.84 ± 1.88 vs 5.09 ± 1.45) (p = 0.001). A value of 5.5 mg/dl was found to be the best cut-off point to diagnoses of early onset neonatal sepsis.
The best way to decrease morbidity and mortality due to sepsis is the early detection and successful treatment [17]. Serum biomarkers for sepsis can generally be classified into pro-inflammatory molecules, endothelial proteins, and molecular patterns/cell surface receptors associated with injury [18].
As regard demographic data, this study showed no statistically significant difference between controls and patients regarding gender distribution, age and maturity.
In the present study, the three most common organisms found among the cultures were staphylococcus aureus, followed by Klebsiella and E. coli.
This was accepted with a study by Shehab El-Din., et al. [19] conducted in three Egyptian Neonatal Network participants' neonatal intensive care units. Coagulase negative staphylococci were predominant isolates followed by Klebsiella pneumonia.
In an agreement with the study of Moges., et al. [20] study in Ethiopia about bacterial etiologic agents causing neonatal sepsis and associated risk factors, Gram positive bacteria were commonly isolated. The commonly isolated bacterial species were S. aureus (40.8%) followed by coagulase negative Staphylococci (21.6%) and K. pneumoniae (15.8%). Similar to Guclu., et al. [2] study, pneumonia was clearly the most common isolate among gram negative bacteria. Staphylococcus aureus was the most common gram-positive isolate. In contrast with the present study, Park., et al. [21] studied cultured bacteria. Enterococcus faecium and Streptococcus were the most common microbes on blood culture. This contrast could be explained by small and insufficient sample size (18 cases).
The comparison between our studied groups according to WBC parameters showed that cases had significantly higher total leukocytic (TLC) count compared to that of controls (15.01 ± 7.78 versus11.63 ± 5.74). This is agreed with a study of Choudhary., et al. [22] about evaluation of platelets and its indices as a marker of neonatal sepsis who found that total leucocytic count was higher in sepsis group than control group with statistical significant difference (p < 0.001).
Moreover, this is similar to Martins., et al. [23] study about Neutrophil-lymphocyte ratio in the early diagnosis of sepsis in an intensive care unit: in which the number of white blood cells in new-borns with high clinical suspicion of sepsis was significantly higher than that in the control group (P = 0.000). The percentage of neutrophils was significantly different in new-borns with high clinical suspicion of sepsis and in controls (P = 0.000).
Although our study group showed lower Platelet-lymphocyte ratio and Neutrophil-Lymphocyte Ratio between cases and controls but without statistically significant difference.
Moreover, this is in an agreement with Shehab El-Din., et al. [19] They studied epidemiology of neonatal sepsis and implicated pathogens, which found that abnormalities in the CBC were found in 66.8% of cases, (22.3%) leucocytosis, (23.2%) neutropenia, and (45.5%) thrombocytopenia.
On the other hand, Elgendy., et al. [24] found that sepsis group had lower Platelet-to-lymphocyte ratio and Neutrophil-Lymphocyte Ratio in his study in the NICU of Menoufia University Hospital for evaluation of hepcidin as a biomarker for neonatal sepsis.
In the present study, cases had statistically significant lower serum uric acid than controls the diagnostic cut-off values of Serum uric acid for neonatal sepsis was 5.5 mg/dl or less.
This agreed with the study of Shalaby., et al. [25] about mean platelet volume and serum uric acid in neonatal sepsis. They showed that septic neonates had statistically lower levels of serum uric acid than the control group. The diagnostic cut-off values of mean platelet volume and serum uric acid for neonatal sepsis were 10.2 fl, and 3.70 mg/dl, respectively.
Moreover, this agreed with Kapoor., et al. [9] study about lipid peroxidation and antioxidants in neonatal septicaemia. The newborns with sepsis had lower Serum uric acid levels. Similarly, an Indian study that was conducted by Aydın., et al. [26] about mean platelet volume and uric acid levels in neonatal sepsis found that the new-borns with sepsis had lower serum uric acid levels.
However, the present study is in disagreement with Hooman., et al. [27] study that showed higher serum uric acid levels served as an additive risk factor in sepsis. That may be due to the different study design and insufficient sample size.
Cases with neonatal sepsis in the present study had significantly lower platelets count (p < 0.05), mean platelet volume (p < 0.05), platelet distribution width (p < 0.05) and platelet crit (p < 0.05).
In the present study, the diagnostic cut-off values of mean platelet volume for neonatal sepsis was 10.2 fl with sensitivity and specificity of 58.3% and 89.8%, respectively.
However, this is in disagreement with Karne., et al. [28] study that reported that elevated mean platelet volume might be used as a marker for screening of sepsis.
Choudhary., et al. [22] reported that high mean platelet volume, high PDW and thrombocytopenia are more common in late onset than early onset sepsis.
Catal., et al. [29] study about mean platelet volume (MPV) may simply predict the severity of sepsis in preterm infants estimated that elevated MPV can be a diagnostic factor along with other findings and can even be utilized for following the course of the sepsis in neonates but, in line with the present study, they didn’t find any correlation between elevated MPV and different kinds of sepsis germs.
Moreover, the present study is in an agreement with Shalaby., et al. [25] study which found that the diagnostic cut-off values of MPV for NS was 10.2 fl.
ROC curve in the present study showed that PWD less than 17.65 demonstrated good diagnostic accuracy in predicting neonatal sepsis with sensitivity of 55% and specificity of 76%.
This disagreed with some authors who reported that septic patients with PDW levels greater than 18% have worse outcome [2]. Moreover, Karne., et al. [28] found 45 out of 103 cases (43.69%) of proven and probable sepsis showed increased PDW value.
The present study demonstrated statistically positive significant correlation between gestational age and uric acid among cases.
This is in agreement with Shalaby., et al. [25] study, SUA showed statistically significant positive correlation with gestational age. While it showed statistically significant negative correlation with CRP. Aydın., et al. [26] also found positive correlations between SUA levels, gestational age (r = 0.20, p = 0.000) and birth weight (r = 0.22, p = 0.000).
Copyright: © 2021 Enas Elnagar., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.