Maria do Céu Pereira Gonçalves1*, Giovanna Marcella Cavalcante Carvalho2, Maria Amélia Porto3 and Márcia Gonçalves Ribeiro3
1Petrópolis Municipal Health Foundation, Neonatal Intensive Care Unit of the Alcides Carneiro School Hospital, Petrópolis, (Rio de Janeiro), Brazil
2Physiotherapy Sector, State University of Rio de Janeiro, Pedro Ernesto University Hospital, Rio de Janeiro, (Rio de Janeiro), Brazil
3Division of Pediatrics, Federal University of Rio de Janeiro, Rio de Janeiro, (Rio de Janeiro), Brazil
*Corresponding Author: Maria do Céu Pereira Gonçalves, Petrópolis Municipal Health Foundation, Neonatal Intensive Care Unit of the Alcides Carneiro School Hospital, Petrópolis, (Rio de Janeiro), Brazil.
Received: April 09, 2021; Published: June 29, 2021
Citation: Maria do Céu Pereira Gonçalves., et al. “The Relationship Between Hyaline Membrane Disease and Primitive Reflexes in Preterm Newborns with a Corrected Gestational Age of 35 Weeks”. Acta Scientific Paediatrics 4.7 (2021): 62-70.
Preterm birth can result in various clinical complications in neonates, including a particularly high prevalence of respiratory disorders. The most common respiratory problems in preterm newborns (PTNBs) are early respiratory discomfort (ERD) and respiratory distress syndrome (RDS), the latter associated with hyaline membrane disease (HMD) in infants born with a gestational age of less than 33 weeks, which can ultimately result in the infant needing to be admitted to a neonatal intensive care unit. However, the relation between these conditions and signs of brain injury has yet to be studied. The aims of this study were to estimate the frequency of RDS/HMD and determine the association between these conditions and abnormal primitive reflex responses and righting reactions in PTNBs with a corrected gestational age (CGA) of 35 weeks. We conducted a descriptive-analytical cross-sectional study using a prospective cohort design. The sample consisted of 450 PTNBs with a CGA of 35 weeks at the time of examination. The physical examination and neonatal data were recorded on two separate forms.
The incidence of ERD was 59.1% (n = 266) and 102 of the infants with this disorder (22.7% of the overall sample) developed RDS/ HMD. The prevalence of RDS/HMD in PTNBs with a gestational age (GA) of less than 28 weeks was 100%. Most infants had an Apgar score of ≥ 7 across all GA groups. The prevalence of abnormal reflex responses and righting reactions in PTNBs with a GA of less than 28 weeks was 76.9%. When neonatal complications considered risk factors for brain injury (intracranial hemorrhage, hypoxemia, acute fetal distress and hyperbilirubinemia) were excluded, there was a significant association between quality of reflex responses and righting reactions and the occurrence of RDS/HMD. Four of the 102 PTNBs who developed RDS/HMD (0.9% of the sample; with a GA of 30 - 32.4 weeks) showed abnormal reflex responses and reactions despite not having any of the complications that are risk factors for brain injury and obtaining one and five-minute Apgar scores of ≥ 7.
Conclusion: In the present study, the results suggest that it is possible to have a relationship between RDS/HMD with abnormal primitive reflex responses and righting reactions in PTNBs with 35 weeks of CGA (0.9% incidence).
Keywords: Premature Newborns; Early Respiratory Discomfort; Hyaline Membrane Disease; Respiratory Distress Syndrome; Primitive Reflexes
Preterm birth can result in clinical complications in preterm newborns (PTNBs) with a gestational age (GA) of less than 34 weeks and weighing under 1500 grams. The incidence of respiratory tract diseases is particularly high in this group [1,2]. The most common respiratory problem is early respiratory discomfort (ERD); however, premature infants born at less than 33 weeks with surfactant deficiency can develop hyaline membrane disease (HMD), which is manifested in respiratory distress syndrome (RDS) [3].
The synthesis of surfactant begins at 20 to 24 weeks of gestation and surfactant reaches chemical maturity at 34 weeks [4,5]. Pulmonary surfactant is secreted by type II alveolar epithelial cells, reducing the air-liquid surface tension and helping prevent tendency to interstitial edema [6].
Surfactant deficiency results in reduced lung compliance and alveolar atelectasis [6,7]. Together, these factors reduce the stability of the alveoli and increase the work of breathing, leading to admission to a neonatal intensive care unit (NICU) to stabilize breathing [5,8].
RDS is one of the most serious and frequent problems affecting PTNBs, accounting for around 50% of neonatal respiratory diseases and 80% to 90% of neonatal deaths [9]. According to the World Health Organization, significant progress has been made in child survival since 1990, with the number of global neonatal deaths declining from 5.0 million in 1990 to 2.4 million in 2019. Most neonatal deaths (75%) occur during the first week of life, and around 1 million newborns die within the first 24 hours. Preterm birth, intrapartum-related complications (birth asphyxia or lack of breathing at birth), infections and birth defects were the leading causes of neonatal deaths in 2017 [10].
It is important to highlight that one of the signs of an intact central nervous system (CNS) and brainstem is the presence of primitive reflexes during the first months of life [11]. When the CNS is damaged, including damage caused by respiratory problems, the infant begins to show patterns of abnormal reflex activity, resulting in abnormal primitive reflexes and righting reactions [11,12].
However, the relationship between RDS/HMD and signs of brain injury has yet to be studied.
The aim of this study was to estimate the incidence of RDS/HMD and determine whether there is a relationship between the disease and abnormal primitive reflex responses and righting reactions in PTNBs with a corrected gestational age (CGA) of 35 weeks.
This study was conducted in a teaching hospital in PetrópolisBrasil in partnership with the municipal health foundation and a federal university.
We conducted a descriptive-analytical cross-sectional study using a prospective cohort design to examine the relationship between RDS/HMD and signs of brain injury in PTNBs with a CGA of 35 weeks on the day of the newborn physical examination.
The necessary sample size was estimated considering a Type I error (α = 0.05) and power level of 95%, resulting in a minimum sample of 450 PTNBs with a CGA of between 35 weeks and 35 weeks and six days.
To select the study participants, the lead researcher (who is a permanent staff member at the hospital) visited the maternity unit three times a week on alternate days to identify eligible PTNBs based on the newborn’s medical records. Data was not collected during the months of January because the lead researcher was on leave.
PTNBs born with a gestational age (GA) of up to 35 weeks and two days, determined using the new Ballard score; PTNBs with a CGA of between 35 weeks and 35 weeks and six days on the day of the physical examination; PTNBs weighing > 1500g on the day of the examination; clinically and hemodynamically stable PTNBs based on the following indicators: respiratory rate (30 - 60 bpm); heart rate (120 - 160 bpm); peripheral oxygen saturation (SpO2 > 89%) without administration of additional oxygen systems, in the moment of the evaluation; absence of signs of respiratory distress/ pain, cyanosis and pallor; PTNBs born in the teaching hospital; parents or legal guardians agreed to participate in the study and signed a written informed consent form.
PTNBs with visible congenital malformations or anomalies identified using imaging techniques; genetic disorders, epilepsy, meningitis, history of trauma, anaemia, hemorrhagic conditions, presence of brain hemorrhage, history of convulsions, acquired immunodeficiency syndrome/human immunodeficiency virus (AIDS/HIV); use of anticonvulsant drugs; use of ventilatory support, total parenteral nutrition (TPN) and/or a central catheter to administer TPN or medication at the time of the physical examination; TORCH infections (toxoplasmosis, syphilis, rubella, cytomegalovirus, herpes virus) identified in the PTNBs or maternal history; PTNBs whose mother was using anticonvulsant drugs (phenobarbital, phenytoin, carbamazepine, valproic acid and others) and/or addicted to drugs.
The data were collected between June 2008 and December 2012. Newborns admitted to the hospital undergo routine periodic neurological examinations to assess primitive reflexes and righting reactions. The mothers of the eligible PTNBs were invited to allow their babies participate in the study, with those who agreed signing an informed consent form. The physical examination was performed by a single previously trained researcher in the intermediate intensive care unit (for PTNBs needing intensive care), nursery (area for weight gain, suction and/or administration of medication) and rooming-in unit (where mothers and infants stay together in the same room after delivery). Relevant clinical information was also collected from the mothers’ and PTNBs’ medical records.
The following steps were performed during each visit: (1) selection of eligible PTNBs; (2) invitation to participate in the study made to the mother/legal guardian; (3) signing of the informed consent form by the mothers/legal guardians who agreed to participate in the study; (4) physical examination of the PTNBs to assess primitive reflexes and righting reactions; (5) interview with the mother/legal guardian to collect maternal data; and (6) collection of clinical information from the mothers’ and PTNBs’ medical records to identify risk factors for brain injury and clinical status.
The data were recorded on two separate forms: one for the physical examination and one for gestational, perinatal and neonatal data.
The data were coded, entered into separate digital worksheets (Microsoft Excel), checked, and exported to the statistical software package SPSS Version 22.0 for Windows (SPSS Inc, Chicago, IL) for processing.
The physical examination was performed using a neurodevelopmental primitive reflex and righting reactions chart [13]. The neurological assessment was performed uniformly on all PTNBs in the sample. The primitive reflexes and righting reactions were tested from head to toe with the infant in the supine position, prone position, sitting position, and upright position.
The independent variables were the risk factors present (Apgar score of ≤ 6, intracranial hemorrhage, hyperbilirubinemia, acute fetal distress and SDR/HMD). The dependent variables were: (1) primitive reflexes (Moro, palmar grasp, plantar grasp, sucking, rooting, Babkin, crossed extension, stepping); (2) righting reactions (Galant, labyrinthine righting, astasia, neck righting and plantar support); (3) pathological reflexes (asymmetrical tonic neck, tonic labyrinthine, positive support reaction). These variables were categorized as follows: “present”, when the reflex responses and reactions were totally consistent with normal motor patterns; “weak”, when the responses were partially consistent with normal motor patterns; “absent”, when the infant failed to show motor response; and “abnormal”, when the infant’s motor patterns were different to those described in the relevant literature.
The physical examinations were performed by a single previously trained examiner from the NICU team when the PTNBs reached a CGA of between 35 weeks and 35 weeks and 6 days. The examinations were performed in a quiet room 30 minutes before feeding so that the responses could be observed under normal conditions without the need to interrupt the test for feeding and to avoid weak responses due to the influence of the sensation of feeling full after feeding.
The primitive reflexes and righting reactions were blind tested with the infant in the fifth state of consciousness and without any prior knowledge of neurological or newborn complications that could lead to a false interpretation of the pattern of reflex responses and reactions [14].
The following descriptive statistics were used to analyze the quality of responses: frequencies, means, standard deviation, prevalence rates, and measures of association between the neonatal variables and results of the physical examination of the primitive reflexes and righting reactions.
The risk factors and quality of the primitive reflexes and righting reactions are described using frequencies presented as percentages. The relationship between the primitive reflexes and righting reactions and RDS/HMD was determined using Pearson’s chi-squared test (X2) with analysis of variance (ANOVA), adopting a significance level of p < 0.05.
The distribution of the neonatal data is described in frequency and association tables. The data were compared using statistical tests to determine differences in means and proportions. Differences between continuous variables were examined using Student’s t-test. The statistical significance of the results for the categorical variables was tested with bivariate analysis using Pearson’s chisquared test (X2). ANOVA was applied to determine the significance of the differences between the means of the continuous variables and different categories of reflex response and righting reaction patterns. A significance level of p < 0.05 was adopted for all associations.
The study was conducted in accordance with National Health
Council Resolution Nº 196/96 and Articles 122 to 130 of the 1988 Code of Medical Ethics. The study protocol was approved by the hospital’s Research Ethics Committee (18 April 2008, Nº 04/08) and by the heads of the maternity unit and neonatal intensive care unit. To safeguard participant anonymity and confidentiality, the medical records were identified in numeric order using Arabic numerals. All participants were informed of the study procedures and signed a written informed consent form.
All PTNBs diagnosed with abnormal primitive reflexes and righting reactions received treatment and follow-up in a secondary care center in Petrópolis-Brasil that offers neonatal and neuropediatric care, physiotherapy and speech therapy services delivered by a multi-disciplinary team.
A total of 450 PTNBs were examined over a period of 4 years and 5 months. Losses occurred in the months of January due to the leave taken by the head researcher and due to the fact that some of the eligible PTNBs were clinically unstable. Table 1 shows the frequency of respiratory comorbidities. The most prevalent disease was ERD, followed by RDS/HMD. Necessary procedures performed on the PTNBs before data collection included oxygen therapy (56.4% of PTNBs) and invasive mechanical ventilation (29.3% of PTNBs).

Table 1: Frequency of respiratory system comorbidities among
the 450 PTNBs.
*RDS/HMD: Respiratory distress syndrome caused by hyaline
membrane disease.
Table 2 shows the stratified frequencies of the 450 PTNBs in relation to GA, RDS/HMD incidence and Apgar scores at one, five, and ten-minutes after birth. The findings show that 13 of the 102 PTNBs who had RDS/HMD were born with a GA of ≤ 28 weeks and that all the infants born at this age developed RDS/HMD. Sixty-one of the 79 PTNBs with a GA of 28.1 - 32.0 weeks had HMD. The frequency of RDS/HMD was highest in this age group (59.8%). The results also show that most PTNBs had Apgar scores ≥ 7 at all intervals across all GA groups.
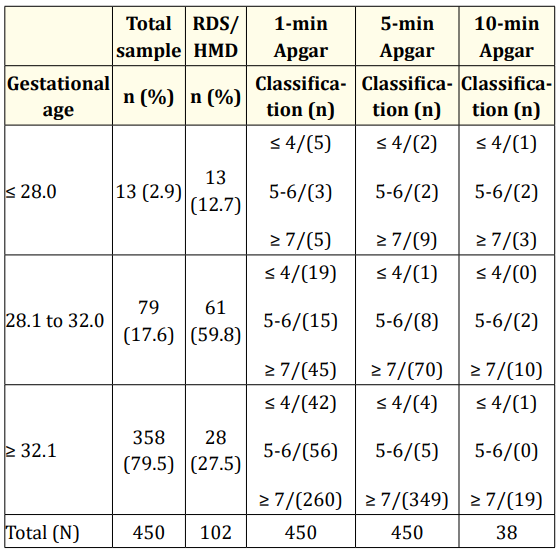
Table 2: Stratified frequency of 450 preterm newborns in relation
to gestational age, RDS/HMD incidence and Apgar
scores at 1, 5, and 10-minutes after birth.
Note 1: Only 38 PTNBs were given the 10-minute Apgar test.
Note 2: Apgar scores were classified as follows: severe asphyxia
(≤ 4), mild asphyxia (5 - 6) and absence of asphyxia (≥ 7).
Table 3 shows the association between GA and normal and abnormal reflex responses and reactions. Abnormal reflex responses and reactions were observed in all GA groups. Prevalence was highest in the ≤ 28 weeks group (76.9%) followed by the ≤ 32 weeks group (64.6%). This difference was statistically significant (p < 0.001).

Table 3: Association between gestational age and reflex responses and reactions.
Table 4 shows that the association between one and five-minute Apgar scores and quality of reflex responses and reactions was statistically significant (p < 0.001). Mean Apgar scores for these intervals were higher among the normal response group than in the abnormal response group: one-minute score 7 ± 1.7 and 5.8 ± 2.3 and five-minute score 9 ± 0.8 and 8 ± 1.5, respectively.
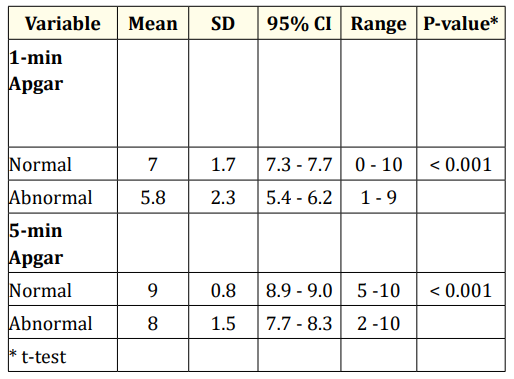
Table 4: Means, standard deviation, CI, range and p-values for one and five-minute Apgar scores and reflex responses and reactions.
Table 5 shows that there was a statistically significant association between all neonatal complications (HMD, intracranial hemorrhage, acute fetal distress and hyperbilirubinemia) and reflex responses and reactions (p < 0.001).
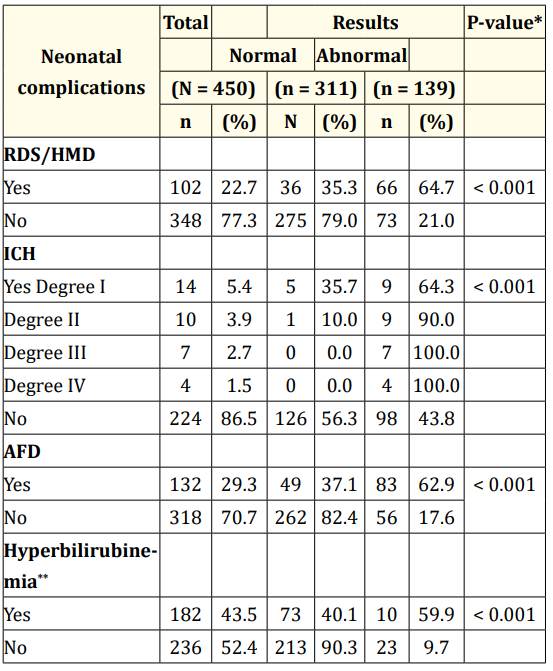
Table 5: Association between neonatal complications and reflex
responses and reactions.
**RSD/HMD: Respiratory distress syndrome caused by hyaline
membrane disease; ICH: Intracranial Hemorrhage; AFD: Acute
Fetal Distress. ***Jaundice: 32 cases ignored.
Table 6 presents the distribution of cases of RDS/HMD with or without the occurrence of intracranial hemorrhage, AFD and hyperbilirubinemia.
Seventy-nine of the 102 PTNBs who had RDS/HMD did not have intracranial hemorrhage. Of these, 47 showed abnormal reflex responses and reactions. Fifty of the 81 cases of RDS/HMD without AFD showed abnormal reflex responses and reactions. Finally, four of the 12 cases of RDS/HMD without hyperbilirubinemia showed abnormal responses, representing 0.9% of the total sample. It is also worth highlighting that these four PTNBs did not have intracranial hemorrhage or acute fetal distress.
Table 7 shows the individual characteristics of the four PTNBs who showed abnormal reflex responses and reactions without the occurrence of risk factors for brain lesion.
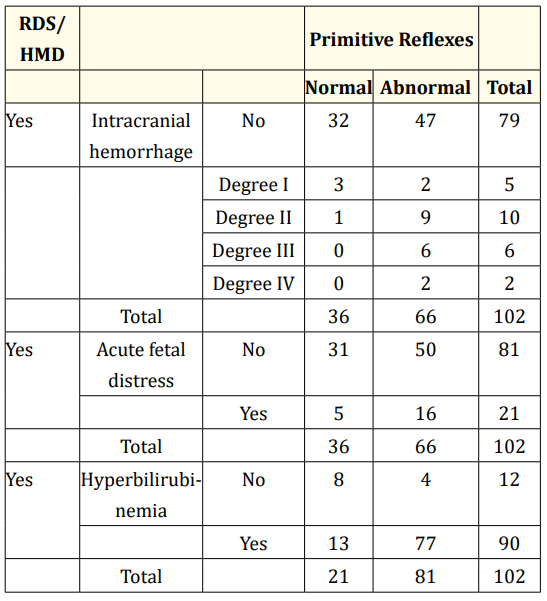
Table 6: Reflex responses and reactions in cases of RDS/HMD
with or without intracranial hemorrhage, acute fetal distress and
hyperbilirubinemia.
Note 1: For the purposes of this study, hyperbilirubinemia was
considered to be TSB > 5.6 mg/dL. Note 2: TSB: Total serum bilirubin. Note 3: For the purposes of this study, acute fetal distress was
considered to be an Apgar score of ≤ 3.
The findings show that these infants had a GA of between 30 and 32 weeks and 4 days, birth weight was adequate for GA, and all PTNBs had one and five-minute Apgar scores of ≥ 7. However, all these newborns developed RDS/HMD, needed invasive mechanical ventilation (IMV) and used surfactants.
Furthermore, when these newborns were assessed at a CGA of 35 weeks, they showed hypertonia in all four limbs, albeit more pronounced in the legs. These infants also exhibited weak abnormal responses for the sucking reflex and asymmetrical tonic neck reflex and abnormal responses for the palmar grasp and Babkin reflexes and labyrinthine and cervical righting and plantar support reactions.
It is worth highlighting that none of the four newborns showed premature rupture of the ovulatory membrane, hypoglycemia, sepsis or respiratory complications (bronchopulmonary dysplasia, atelectasis and pneumonia).
All the PTNBs in the sample (N = 450) showed the same pattern of response for the primitive reflexes/righting actions Moro, voracity, plantar grasp, Galant and crossed extension. The pathological reflexes tonic labyrinthine and positive support reactions were not observed in the sample, even in the PTNBs who showed abnormal responses.
RSD/HMD showed an association with abnormal primitive reflexes and righting reactions. In addition, when the risk factors for brain injury (intracranial hemorrhage, AFD and/or perinatal asphyxia and hyperbilirubinemia) were excluded, four PTNBs exhibited abnormal reflex responses and righting reactions, suggesting that RDS/HMD alone can cause cerebral palsy. Considering that at some point during the stay of the PTNB in the neonatal intensive care unit, episodes of hypoventilation/hypoxemia may occur.
RSD/HMD incidence decreased with increasing GA. The disease generally occurs in around 50% of babies born between 26 and 28 weeks and in 25% of babies born between 30 and 31 weeks [15]. With regard to the 102 PTNBs who developed RDS/HMD, 12.7% had a GA of less than 28 weeks, 59.8% had a GA of between 28.1 and 32 weeks, and 27.5% had a GA of ≥ 32.1 weeks, corroborating the findings of previous studies.
The incidence rates observed by the present study may be explained by the absence or deficiency of surfactant, particularly the four surfactant proteins (SPs) expressed by the respiratory epithelial cells, SP-A, SP-B, SP-C and SP-D. SP-A and SP-D play an important role in pulmonary host defense [16], while SP-A is important for the organization and function of the surfactant complex that regulates the recycling and secretion of surfactants. SP-B and SP-C have surface tension reduction properties and are important for the absorption and spreading of surfactant [17,18]. It is well-known that the primary function of pulmonary surfactant is to reduce the air-liquid surface tension in the lung, preventing the alveoli from collapsing and thus facilitating breathing [6]. Additionally, SP-A and SP-B create dipalmitoylphosphatidylcholine (DPPC) enriched domains that can be readily adsorbed to create a DPPCrich monolayer at the interface [18]. Our hypothesis is that, as a result of this biochemical and biophysical dysfunction, the lung is exposed at some point to hypoventilation, which can lead to systemic hypoxemia including the CNS.

Table 7: Characteristics of the 4 PTNBs without the occurrence of risk factors for brain injury who showed abnormal reflex responses
and reactions when assessed at a CGA of 35 weeks.
NE: Not Assessed; IMV: Invasive Mechanical Ventilation; HMD: Hyaline Membrane Disease; PTNB: Preterm Newborn. Note: none of the
PTNBs were given the 10-minute Apgar test.
As other authors show, the five-minute Apgar score is commonly used to predict asphyxia, hypoxic-ischemic encephalopathy and cerebral palsy [19,20]. The present study observed a statistically significant association between one and five-minute Apgar scores and the quality of reflex responses and righting reactions. The PTNBs who showed abnormal responses obtained lower one and five-minute Apgar scores than those who exhibited normal response. Apgar scores of < 7 in the infants who showed abnormal responses characterize the presence of hypoxemia, leading to a high risk of hypoxic-ischemic syndrome. Hypoxemia can be identified by the presence of abnormal reflex responses and righting reactions, as observed in this study. In contrast, the four PTNBs had one and five-minute Apgar scores of ≥ 7, suggesting ERD with hypoventilation associated with HMD, given that all the infants developed RDS/HMD and needed mechanical ventilation and the use of at least one dose of surfactant. This has implications for clinical practice, since when one and five-minute Apgar scores reach ≥ 7, the health team feel more reassured in relation to neuromotor behavior. The clinical examinations of the four PTNBs revealed hypertonia, reinforcing the importance of monitoring oxygenation in preterm newborns in order to avoid episodes of hypoxemia and hypoventilation.
It is worth highlighting that the two main causes of tissue hypoxia are low blood flow to tissues and/or low blood oxygen levels [6]. A severe restriction of oxygen supply leads to deterioration in the function of organs and, particularly, neurons [21-23]. It is interesting to note that, despite the fact that prematurity is one of the key predisposing factors in the etiology of HMD [24,25] and that the disease is characterized by acute lung injury with pulmonary edema and non-hydrostatic hypoxemia [26], the four PTNBs who showed abnormal reflex responses and reactions did not have any complications other than the fact that they were premature and developed RDS/HMD. Due to prematurity and the immature central nervous system, even mild hypoxemia can have a negative effect on sensorimotor brain function [27]. It is therefore possible that the four infants had hypoxemia caused by hypoventilation or poor perfusion, leading us to believe that the presence of pulmonary impairment may have brought about hypoventilation with low cerebral oxygenation, manifested in abnormal reflex responses and righting reactions.
The instrument used to assess the quality of primitive reflexes and righting reactions is an adequate test for screening abnormal postural reactions, particularly for the identification of risk of cerebral palsy [28]. Some authors observed that certain primitive reflexes and righting reactions were absent in high-risk infants with abnormal reflexes indicating cerebral palsy. In addition, they found that the absence of the Moro or plantar grasp response in infants may be a predictor of the development of cerebral palsy [29]. The results of the present study corroborate these findings. Cerebral palsy can be detected in the first six months of life by performing a physical examination of primitive reflexes and righting reactions [30].
In the present sample, the results suggest that it is possible to have a relationship between RDS/HMD with abnormal primitive reflex responses and righting reactions in PTNB with 35 weeks CGA, suggesting that perhaps the RDS/HMD may be one of the etiological factors of cerebral palsy, whereas at some point may be to occur episodes of hypoventilation/hypoxemia.
SatO2 was not collected daily from the medical record during the study period, which could have allowed us to correlate the variation in SatO2 rates with the abnormal responses found in the neurological examination of the PTNBs in the study.
Copyright: © 2021 Maria do Céu Pereira Gonçalves., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.