Lysandro Pinto Borges1*, Adriana Gibara Guimarães1, Fernanda Camargo Mendonça de Araujo1, Jessiane Bispo de Souza1, Daniela Raguer Valadão Souza2, Aline Fagundes Martins2, José Melquiades de Rezende Neto2, Luis Eduardo Cuevas3 and Ricardo Queiroz Gurgel4
1Department of Pharmacy and Clinical Analysis, Federal University of Sergipe, São Cristóvão, Sergipe, Brazil
2Department of Education in Health, Federal University of Sergipe, Lagarto, Sergipe, Brazil
3Department of Clinical Sciences, Liverpool School of Tropical Medicine, Liverpool, United Kingdom
4Department of Medicine, Federal, Post-Graduate Programs in Parasitic Biology and Health Sciences, Federal University of Sergipe, Aracaju, Sergipe, Brazil
*Corresponding Author: Lysandro Pinto Borges, Department of Pharmacy and Clinical Analysis, Federal University of Sergipe, São Cristóvão, Sergipe, Brazil.
Received: March 01, 2021; Published: May 15, 2021
Citation: Lysandro Pinto Borges., et al. “Main Clinical Sequels in Patients Affected by Covid-19: A Systematic Review”. Acta Scientific Paediatrics 4.6 (2021): 42-50.
In view of the appearance of several sequelae due to infection by COVID-19 and the need to seek knowledge about this new disease, this review aimed to identify the main clinical sequelae in patients affected by COVID-19. The literature search was performed in the PubMed, SCOPUS, Web of Science and Science Direct databases and were included in a total of 16 studies. The main sequelae found were cardiac, neurological and pulmonary, other sequelae were also found such as kidney injury, gastrointestinal symptoms, joint pain, fatigue, excessive sweating and hair loss, however, in smaller proportions. These data provide a health team, during specific treatments of COVID-19, to avoid these sequelae, enabling health professionals to develop new treatment strategies, as well as more assertiveness in the diagnosis and thereby optimize the survival of the patient.
Keywords: COVID-19; Kidney Injury; Gastrointestinal Symptoms; Joint Pain; Fatigue
Since the first report of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection on December 19, 2019 in China, about 84 million cases have been diagnosed worldwide, with more than 1 million deaths from the disease [1]. Now, most of the world is experiencing the second wave of pandemic, which has shown different severity and characteristics of the first [2].
SARS-CoV-2 infections can be asymptomatic or oligosymptomatic. Among symptomatic patients, most people develop mild (40%) or moderate (40%) disease, 15% manifest severe disease, requiring oxygen support, and 5% present the most critical state of the disease, with complications respiratory, sepsis and septic shock, thromboembolism, acute kidney injury, cardiac injury and/ or multi-organ failure [3].
Although pathophysiology has not yet been clearly elucidated, it is known that virus attacks several organs of the human body, causing important damage where at least 15% of the carriers of the infection progress to severe clinical manifestations and those who survive are left with some type of sequel [4]. For this reason, World Health Organization (WHO) recommends that member states address the challenge of to characterize and comprehensively manage the complications and sequelae of COVID-19, also guaranteeing the continuity of follow-up and assistance to patients who have had sequels of the disease [4].
In view of the emergence of several sequels resulting from the infection by COVID-19 and the need to seek knowledge about this new disease, this review aimed to map the main cases of clinical sequelae in patients diagnosed with COVID-19.
This review follows the model proposed by PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyzes) [5]. A systematic review is defined as “a review of the evidence on a clearly formulated question that uses systematic and explicit methods to identify, select and critically appraise relevant primary research, and to extract and analyze data from the studies that are included in the review”. The methods used must be reproducible and transparent [6].
The first step in developing a systematic review is to formulate the research question, which must be formulated in a systematic way. To fulfill these requirements, it is necessary to we need to have a good overall knowledge of literature, to question the truths that are considered absolute and to have expertise in the area, in order to understand the aspects to be examined [5]. To define the guiding question of the systematic review, the PICOTT strategy was used, shown in the table 1.
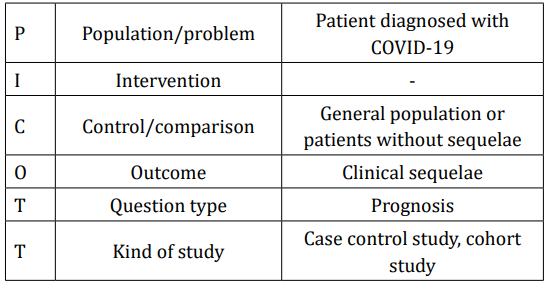
Table 1: Formulation of the question.
Due to the several cases found in the literature that brings the problem of sequels left by covid-19, a scope review was then carried out with the following question: What are the main clinical sequelae in patients with COVID-19?
The literature search was performed in PubMed, SCOPUS, Web of Science and Science Direct databases. The strategy was adapted according to the protocol of each database (Table 2), until the deadline of November 11, 2020, with restrictions on the language of publication and the year 2020. The selection of studies was performed using an online platform for reviews - Rayyan QCRI to assist in the quantification, selection, elimination of duplicate studies and conflict resolution of those selected.
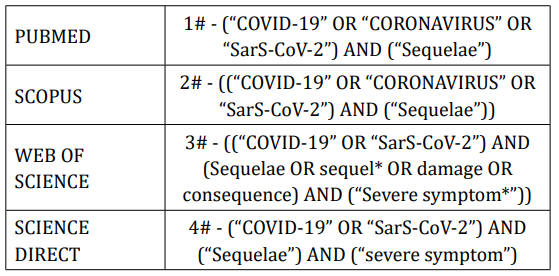
Table 2: Search strategy descriptors.
According to the inclusion criteria, studies were included that reported on the sequelae in patients diagnosed with COVID-19, as well as dated only in the year 2020, published in English, those that had information on post-infection sequelae and that reported diagnosed cases according to the World Health Organization (WHO) for SARS-CoV-2 infection [7].
The exclusion criteria were as follows: duplicates, editorials, comments, opinion articles, communications, not available, letter to the editor and other reviews; without presentation of methodology; and with incomplete information or without definition of the sequelae after infection by SARS-Cov-2 and patients who were not diagnosed by the rRT-PCR method. The entire data extraction process took place according to the analysis process described in figure 1.
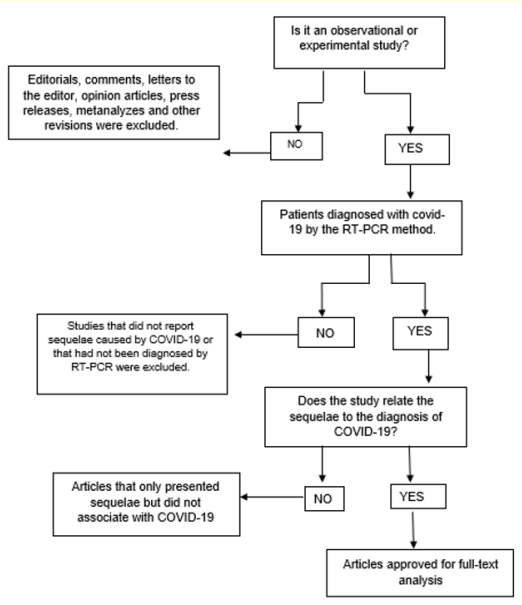
Figure 1: Process for analyzing titles and abstracts.
Two reviewers independently analyzed the search results and taking into account the titles and abstracts, they raised the most relevant studies for reading the full text. The relevant studies were then read in full and following the eligibility criteria were included in the review. A third researcher resolved disagreements throughout the evaluation process.
The data were extracted in duplicate and the disagreements were resolved by consensus. From the included studies, information was extracted on the various types of reported sequelae, postinfection, such as: Sequelae in the cardiovascular system, sequelae in the respiratory system, neuropsychiatric sequelae and other types found.
After search in databases, 899 articles were founded. After restricting by year and language of publication, 899 articles remained. With the resolution of the duplicates and then the full reading of the remaining articles, 16 studies were included, as can be seen in the final reference portfolio as shown in table 3 and figure 2.
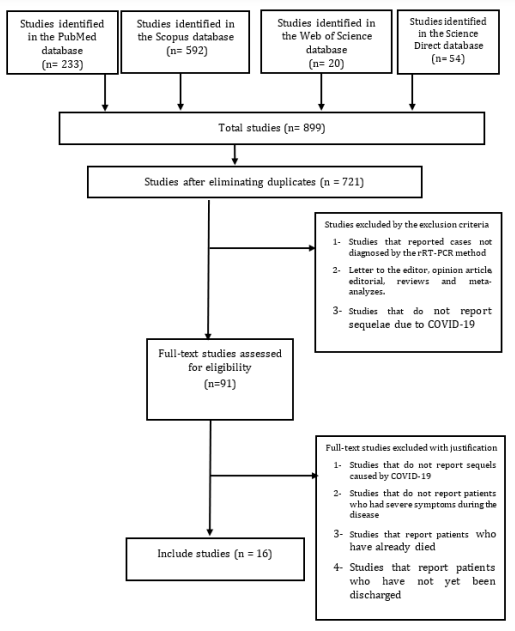
Figure 2: Flow diagram.
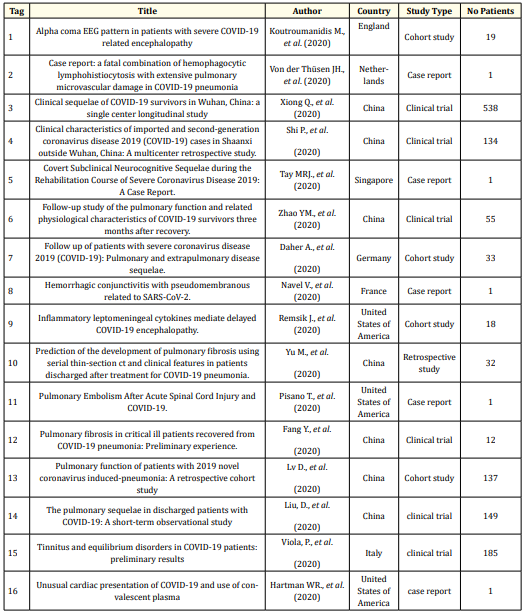
Table 3: Final reference portfolio.
Regarding the countries that have published the most articles related to this topic, we could identify China (7), United States of America (3), England (1), Netherlands (1), Singapore (1), France (1), Germany (1), Italy (1), conformed, indicating the heterogeneity of the studied population. Interest in the topic may be linked to the growing demand from patients who have been affected by the disease and presenting severe sequelae caused by COVID-19. About design of included studies, most were clinical trials (7), followed by report case (5) and cohort studies (4).
The total number of patients in the sample was 1298, with an average of 86.5 ± 140.3 patients per study. The average age was 52.4 ± 8.4 with a population tending to be elderly and adult. Of the most common comorbidities, hypertension prevailed with 12.2%, followed by Diabetes with 5.8% of patients, chronic kidney disease with 5.32%, respiratory diseases with 3.2% and cardiovascular diseases with 2.7%. The number of men and women proved to be balanced, with the majority being male (50.6%).
Regarding the sequelae found, the most common were those involving the respiratory tract with 8 (50%) studies citing some type of respiratory consequence due to COVID-19 ranging from mild sequelae such as cough and expectoration, to more complex ones such as pulmonary fibrosis [9,10,13,14,17,19-21]. Neurological sequelae were the second most cited with 5 (31%) and involved symptoms such as severe encephalopathy and disorders of the central nervous system (CNS) [8,10,12,18,22]. Sequelae related to the cardiac system and ocular sequelae, from other organs and more general symptoms, were also found [10,11,15,23]. Figure 3 represents a word cloud of these main sequelae found and figure 4 possible signs caused by sequelae left by COVID-19.
Excessive systemic inflammation caused by COVID-19 can cause damage to the myocardium and this is due to the high inflammatory response caused by the excessive release of associated cytokines. One of the important factors that contribute to the onset of cardiac sequelae is pre-existing cardiovascular disease in the patient, as well as primary damage to the cardiac myocyte by direct viral infection and the increased demand caused by hypoxia associated with severe infection and respiratory failure [23].

Figure 3: Word cloud showing the main sequelae found.
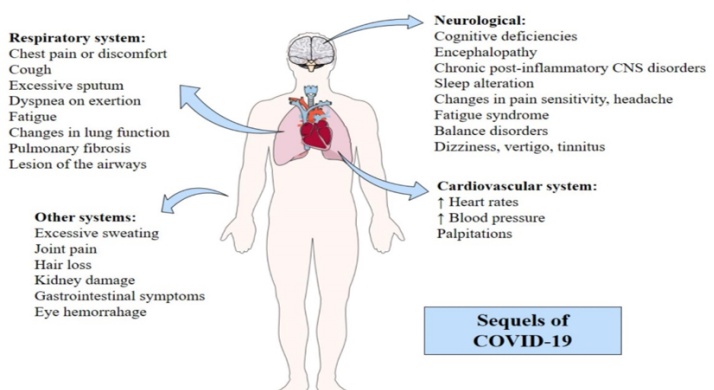
Figure 4: Possible signs caused by sequelae left by COVID-19.
A longitudinal study, conducted in Wuhan, China, with 538 patients who had COVID-19, reported cardiovascular sequelae in seventy survivors who had significant cardiovascular symptoms 3 months after discharge. Of the 60 people who reported heart rates at rest significantly higher than before they had COVID-19, 45 had increases in heart rate at rest during hospitalization that were still present. Twenty-six patients reported occasional palpitations. Seven patients said they were recently diagnosed with high blood pressure and now need medication to lower blood pressure [10].
Pulmonary sequelae were the majority in the studies found. These sequelae have inconvenient consequences for patients, as demonstrated in the study by Xiong., et al. [10] who reported that even after 3 months of hospital discharge, 39% of patients had pulmonary symptoms such as chest pain or discomfort, cough and excessive sputum, but which improved over time. Zhao., et al. [13] also found similar symptoms in their research, such as dyspnea on exertion, as well as cough, sputum and fatigue after the 3-month follow-up after hospital discharge and in 14 of the 55 patients analyzed, they presented changes in lung function. These studies reveal that there may be more chances of persistent lung injury in critically ill patients than in non-severe patients [11,13,19].
The possibility of the formation of pulmonary fibrosis was also mentioned, Yu., et al. [17] followed up 14 patients who developed this sequel and mentions that this group has a longer hospital stay (19.5 days) than the comparative group of 18 patients who did not have pulmonary fibrosis (10 days). In Liu., et al. [21] 54.4% of the patients found fibrous stripes as well as findings of ground-glass opacity (GGO) in radiological images and thickening of the adjacent pleura.
The study by Lv., et al. [20] carries out an evaluation with 137 patients, submitting them to pulmonary function tests after two weeks of hospital discharge. The study demonstrates less subjective results as its studies already mentioned, the results of this article demonstrated that the majority of patients exhibited restrictive ventilatory dysfunction and small lesion of the airways, indicating that the virus causes more damage to the pulmonary alveoli than to the airways.
In a case report by Tay., et al. [12] it was found that a patient's Montreal Cognitive Assessment (MoCA) score revealed cognitive deficiencies in several domains: visuospatial function (1/5), appointment (3/3), attention (4/6), language (1/3) abstraction (0/2), memory and orientation (5/6). In view of the grossly abnormal MoCA, a brain magnetic resonance was performed and this revealed several focal abnormalities with acute margins limited to the depth of white brain matter bilaterally with low signal intensity in T1-weighted MRI images, high signal in T2-weighted images and decreased signal in fluid attenuated inversion recovery (FLAIR).
This suggests that cognitive deficiencies were found especially in the domains of processing speed, memory, visuospatial processing and executive function that correlated with the deep frontal white matter involved. Deficiencies in language processing and nomenclature may be related to the frontal lobe of the dominant (left) hemisphere or memory recovery.
A retrospective observational study conducted at a university hospital in London, 19 patients reported on the Electroencephalogram (EEG) pattern of alpha coma in patients with severe COVID19-related encephalopathy. EEG has been proven to validate a clinical diagnosis of COVID-19-related encephalopathy in 13 patients. It was also found that the 13 patients with EEG characteristics of COVID-19-related encephalopathy had a more severe disease than those without encephalopathy [8].
There are reports in studies that correlate the Central Nervous System (CNS) and the Peripheral Nervous System (PNS) in COVID-19 causing chronic post-inflammatory CNS disorders, adversely affecting sleep, pain sensitivity and energy, which would lead to the development of fatigue syndrome [14].
The conduct of a multicenter study that included 15 Italian hospitals, found the presence of sequelae in 185 patients and found that Thirty-four patients reported balance disorders after the diagnosis of COVID-19. Of these, 32 patients reported dizziness and 2 reported acute bouts of vertigo. Forty-three patients reported tinnitus and 14 reported tinnitus and balance disorders [22].
In the studies there were also reports of other sequelae found, Xiong., et al. [10] reports cases of excessive sweating (127/538), sleep disorders (95/538), joint pain (41/538) and hair loss (154/538) at check up in patients after 3 months of discharge hospital. The laboratory findings of Shi., et al. [11] suggest kidney damage caused by bile duct cell function. Other common symptoms such as headache, fatigue and tiredness, cough and sputum have also been reported [13].
One of the known symptoms during COVID-19 is gastrointestinal symptoms. In a cohort study carried out with 55 patients, even after 3 months of hospital discharge, symptoms related to the gastrointestinal tract were still observed in 22 patients [13].
These symptoms may be related to the mechanism of action of the virus by means of the angiotensin-converting enzyme 2 (ACE2) [24]. Evidence of ocular complications was also found in severe patients with COVID-19. A case reported by Navel, Chiambaretta and Dutheil [15] of a 63-year-old man presenting a case of eye hemorrhage as a sequel to COVID-19 leads the attention of the intensive care unit team to eye symptoms to prevent these sequelae.
The evaluation of the methodological quality of the studies was carried out through the Joanna Briggs Institute Critical Appraisal Checklist for cross-sectional studies, case-control studies, case reports, clinical cases and cohort [25]. Two reviewers blindly assessed the methodological quality of the articles and the disagreements were resolved by consensus.
Regarding the analysis tool of the Cohort studies, it has 11 items, and of the studies analyzed, one completed ten items [19], five completed nine items [8,13,14,17,20] and one completed seven items [16], with regard to the clinical trial study, the tool has ten items, of which the study completed eight [22], in case report studies the tool has eight items, and all studies in this class completed the eight items [9,12,15,18,23], in the case of control case studies, the tool consists of ten items, with one study covering all items [11] and the other study covering nine items [10] and finally, the cross-sectional study that used the tool with eight items and completed six [21].
The possibility of mapping the sequelae in patients who had COVID-19, can help in the implementation of new personalized treatments aimed at the rehabilitation of convalescent patients. Thus, this review present the possible sequels that post-COVID patients may have in respiratory, cardiovascular and nervous systems, in addition to those found in other systems.
In this review, we verified that sequels in the respiratory system are very common, as pain or discomfort in the chest, cough with excessive expectoration and some patients may still experience relevant fatigue [13]. Severely ill patients are more susceptible to having these types of sequelae than those considered non-severe [19]. There are reports that the virus causes more damage to the pulmonary alveoli than to the airways [19].
In fact, when SARS-CoV-2 infects lung cells, starts a cascade of events mediated by virus ligation to ACE2 and release of damageassociated molecular patterns (DAMPs, including ATP). After, proinflammatory cytokines and chemokines released by the tissue induces cells migration, as monocytes, macrophages and T cells, promoting further inflammation, causing overproduction of proinflammatory cytokines, which eventually damages the lung infrastructure, microvascular thrombosis and hemorrhage [26,27].
Besides, the resulting cytokine storm can circulates to other organs, leading to multi-organ damage, with several extra-pulmonary manifestations [28] as in the cardiovascular system [23]. Other studies also report patients with significant cardiovascular symptoms 3 months after discharge with significantly higher resting heart rates than before COVID-19, also had palpitations and some patients were diagnosed with high blood pressure after infection and now need anti -hypertensive [29]. Cardiovascular manifestations presented by COVID-19 patients has been attributed to damage inflammatory induced by cytokine, as IL-6, and downregulation of angiotensin-converting enzyme 2 (ACE2) induced by SARS-CoV2, causing myocarditis, vascular inflammation, metabolic changes, venous thromboembolism. microvascular dysfunction, acute myocardial injury, heart failure and arrhythmia [30].
Moreover, neuropsychiatric damage has been reported in many studies [8,12,14,22]. There was impairment in the visuospatial function, in the ability to nominate, pay attention, in language, abstraction, memory and orientation [12], affecting a considerable number of patients [31]. These alterations are induced by several mechanisms, as strong neuroinflammation generated by the host's immune response against the virus, hypoxia injure with metabolism changes, by ACE2 pathway and direct infection injury, which can promoted infectious toxic encephalopathy, viral encephalitis and cerebrovascular disease [32].
Further, were founded other types of damage in patients who had COVID-19. Kidney injury were reported and has been associated with damage of tubular epithelial cells caused by SARS-CoV-2 antigens accumulated in the kidneys, by ACEII-dependent pathway, during infection phase [33]. In fact, Pei., et al. [34] suggested that acute tubular necrosis (ATN) is the main cause of AKI in COVID-19. Gastrointestinal symptoms also are a reality and this may be associated with modulation of angiotensin-converting enzyme 2 (ACE2), inflammation strong and changes in intestinal flora [13,24,35]. Musculoskeletal manifestations as joint pain and fatigue also has been reported in literature, as consequence of systemic inflammation [36]. Finally, excessive sweating and hair loss, can be associated with metabolic and dermatological changes, and still need more studies.
In this review, it was evident that respiratory, cardiovascular and neurological sequelae are the most common among patients with COVID-19. With these data, it is possible to map the main sequelae of this disease, enabling health professionals to develop new treatment strategies, as well as more assertiveness in the diagnosis and, thus, to optimize the patient's survival.
We thank the staff at the Department of Medicine and Pharmacy (LaBiC) of UFS, and acknowledge financial support received from the Ministério Público do Trabalho, Ministério Público Federal and Ministério Público Estadual.
Copyright: © 2021 Lysandro Pinto Borges., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.