Edwin Dias1,2* and Shwetha N3
1Professor and Head, Department of Pediatrics, Srinivas Institute of Medical Sciences and Research Centre, Mangalore, Karnataka State, India
2Adjunct Research Professor, College of Allied Health Sciences, Srinivas University, Mangalore, India
3Final Year Pharm D., Srinivas College of Pharmacy, Valachil, Mangalore, Karnataka State, India
*Corresponding Author: Edwin Dias, Professor and Head, Department of Pediatrics, Srinivas Institute of Medical Sciences and Research Centre, Mangalore, Karnataka State, India.
Received: April 26, 2021; Published: May 15, 2021
Citation: Edwin Dias and Shwetha. “Recent Approaches in the Management of Difficult to Treat Asthma in Pediatric Population - A Brief Review”. Acta Scientific Paediatrics 4.6 (2021): 24-35.
Introduction: Pediatric patients with difficult-to-treat asthma experience a heavy burden of exacerbations, symptoms, therapeutic failure, adverse drug reactions, and increased health care costs. Frequent cough, dyspnoea, chest tightness, and wheeze interfere with normal daily activities, sleeping, overall quality of life, and education of children. Difficult-to-treat asthma affects a small group of children with asthma but represents a challenging mix of misdiagnosis or incorrect diagnosis, multiple co-morbidities, severe airway pathophysiology, inadequate self-management, and treatment complications. Thus management of such cases requires beyond pharmacotherapy of asthma because patient-related and disease-related domains need to be considered first. Therefore, prevention of asthma exacerbations is an essential goal in difficult-to-treat asthma therapy and requires more focused individualized treatment that involves the elimination of risk factors, treatment of co-morbidities, treatment with anti-asthmatics, and improving medication adherence.
Methodology: A brief review of all the relevant standard articles was conducted.
Result: The study involved a brief review on assessment of risk factors in the pediatric population with difficult-to-treat asthma and pharmacological management i.e. optimal use of long-acting muscarinic antagonist (tiotropium), and biological monoclonal antibody treatment (Omalizumab, Mepolizumab, Dupilumab, and Benralizumab) which were found to be safe and effective in the pediatric population.
Conclusion: Management of difficult-to-treat asthma in the pediatric population requires the elimination of modifiable risk factors, treatment of co-morbidities, and treatment of exacerbations with newer agents which were found to be effective in reducing hospitalizations and frequent Emergency Department visits. Thus individualized treatment must be preferred in these patients.
Keywords: Difficult-to-Treat Asthma; Pediatrics; Risk Factors; Recent Approaches; Management
A list of abbreviations are mentioned in the following table 1.
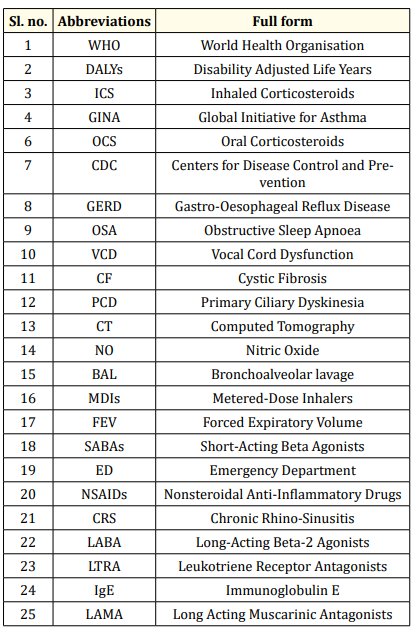
Table 1: List of abbreviations used in this study.
Asthma is a multi-factorial and complex chronic inflammatory disease of the airways characterized by reversible airflow obstruction and airway hyper-responsiveness [1,2]. This is one of the major non-communicable diseases that affected more than 339 million people globally as per the Global Burden of Disease Study 2016 [3]. As per a systematic study conducted by the World Health Organisation (WHO), there were 417,918 deaths due to asthma at the global level and 24.8 million Disability Adjusted Life Years (DALYs) attributable to Asthma in 2016 [4].
Most of the children diagnosed with asthma achieve optimal symptom control when treated with low-to-medium doses (<500 mcg/day fluticasone equivalents) of inhaled corticosteroids (ICS) [5]. But in case of difficult to treat asthma where asthma is still uncontrolled despite GINA (Global Initiative for Asthma) Step 4 or 5 treatments (e.g. medium or high dose inhaled corticosteroids (ICS) with a second controller i.e. maintenance Oral Corticosteroids (OCS)), or that requires such treatment to maintain good symptom control and minimize the risk of exacerbations. It does not mean a ‘difficult patient’, rather it just means ‘difficult-to treat’ because of modifiable risk factors such as incorrect diagnosis, incorrect inhaler techniques, non-compliance, psychological factors, environmental factors or any other co-morbidities [6,7].
Childhood asthma is the most common worldwide disease, imposing a huge burden on the patient, their family, as well as society, include decreased quality of life for the patient and their family, as well as high costs for society [8]. In developed countries, the healthcare expenditures for asthma are 1-2% of the total healthcare costs [8].
As per the report of Centers for Disease Control and Prevention (CDC) 2016, the prevalence of asthma in children aged 5 to 11 years and 12 to 17 years is 9.6% and 10.5% respectively with an overall prevalence of asthma in children under 18 years old in the US is reported as 8.3% [9]. According to an observational, crosssectional, two-phase, multicentre study conducted in 30 hospitals in Spain by Plaza-Martín AM and team the prevalence of difficultto-treat asthma was 24.2% among12,376 asthmatic children [10].
Recurrent exacerbations are one of the major causes of morbidity and medical expenditure in patients with difficult-to-treat asthma [10]. In the majority of pediatric patients, asthma can be well controlled with the help of simple regimens of inhaled drugs. But, some patients with difficult-to-treat asthma suffer from frequent exacerbations of asthma resulting in days of absence from school, the need for emergency care or hospitalization [11,12].
In difficult-to-treat asthma, optimal symptom control cannot be achieved due to several factors that are independent of the disease such as incorrect diagnosis, multiple co-morbidities, poor medication adherence, poor inhalation techniques, psychological or environmental factors [13,14].
It is very essential to remember that “all wheezes are not the symptoms of asthma”. According to a study, in 12-30% of cases, “uncontrolled asthma” was another condition, which was misdiagnosed [15]. Most conditions that may be mistaken for asthma are congestive heart failure, GERD (Gastro-oesophageal reflux disease) obstructive sleep apnoea (OSA), or vocal cord dysfunction (VCD) many others [16,17].
When there is a lack of response to standard therapy, the diagnosis of asthma must be enquired. If asthma control is difficult to achieve, differential diagnostics of conditions with symptoms that may be similar to those of asthma should be intensified. Clinical criteria and differential diagnosis in children with wheezing are described in table 2 and 3 [17].
Once the diagnosis of asthma is confirmed, it is important to ensure adherence to the medication regimen as non-compliance is one of the major problems impairing achievement of the optimal asthma control in children [14,16]. In children with difficult to treat asthma this is particularly necessary because non-adherence to medication is a confounding factor which is responsible for poor clinical outcome [18]. According to a study, 10 to 46% pediatric population diagnosed with asthma were poor adherent to medications and even more so with Metered-Dose Inhalers (MDIs) compared with oral medications [19]. As per one more study, low-compliant patient had significantly worse lung function parameters (post-bronchodilator FEV1 75.4 vs. 84.3, p < 0.05), higher chances of ventilation disorders due to asthma (19.2% vs 2.6%, p < 0.05) and more sputum eosinophil counts (0.66% vs. 0.54%, p = 0.05) [20].

Table 2: Clinical conditions need to be evaluated to avoid incorrect diagnoses in children with wheezing.

Table 3: Time for high index suspicion for differential diagnosis in children with wheezing.
Several reasons exist for poor medication adherence in children and adolescents including complex treatment regimen (difficulties in using an inhaler, multiple inhalations per day, and use of several different types of inhaler), unintentional factors (such as being forgetfulness, absence of a daily routine), and intentional factors (wrong perception that treatment is not necessary, denial, embarrassment, inconvenience, fear of side effects, treatment cost and laziness) [21].
Sometimes, if the patients are compliant, the use of improper inhaler techniques may prevent the appropriate delivery of the drug. Not only this, incorrect inhalation technique could increase the risk of both disease exacerbations as well as the adverse effects of treatment [22]. It is estimated that most of the patients diagnosed with asthma (up to 70-80%) incorrectly use their inhalers and are not aware of the errors they make [14]. These types of activities not only hinder the drug to reach steady-state concentration but also prevent the achievement of optimal clinical outcomes.
Even though many pediatric patients diagnosed with asthma function well, it has been observed that some of them have a higher risk of internalizing emotional and behavioural problems such as anxiety and depressive symptoms [23,24]. This disease burden may lead to behavioural problems such as difficulties in separation or individuation from parents and concomitant anxiety [24]. These psychosocial factors not only trigger the exacerbation of asthma through neuro- endocrine and immune mechanisms, but also may lead to poor adherence, poor disease control and asthma management, and poor functional health status [25,26].
Patients diagnosed with asthma and co-morbid depressions are very difficult to treat. Hence, it is necessary to address and treat the depression before there can be any success with asthma therapy for this population [27].
Parents play a crucial role in the social, autonomy, and overall development of their children. When parents establish a caring, supportive and positive environment, this will have a positive impact on developmental outcomes for especially children growing up with a disease [28].
Some studies have indicated that parental stress is one of the potential cause and consequence of disease activity, asthma control and quality of life, and behavioral problems in children and can exert an influence on the disease through poor treatment adherence or worsening of the patient condition (through physiological stress response systems such as the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis) as to compliance with asthma management still largely depends upon caregivers in children [29-33].
For children with difficult-to-control asthma exposure to allergens or other triggers can exacerbate the clinical signs and symptoms of asthma. Along with pollens, cold conditions, and dust mites, microbial volatile organic compounds released from excess indoor mold growth and water-intruded areas are also increasingly being recognized as important irritants triggering asthma [34]. Thus identifying and eliminating these factors may help with asthma management [16].
Frequent and overuse of SABAs causes down-regulation of beta receptors and lack of response leading in turn to greater use [35]. Sometimes overuse may also be habitual. A study revealed that dispensing of more than 3 canisters per year i.e. average of 1.5 puffs per day or more is associated with increased risk of hospitalization or Emergency Department (ED) visits independent of the severity [36]. One more study revealed that dispensing of more than 12 canisters per year (once a month) increases the risk of death [37].
Several other factors such as concomitant diseases (GERD, obesity, allergic rhinitis or chronic rhino-sinusitis, etc), poor socio-economic conditions, and drug history of nonsteroidal anti-inflammatory drugs (NSAIDs) and β-blockers can be significant unidentified precipitators of difficult to treat asthma [38,39].
Difficult-to-treat asthma can be defined when asthma is not controlled despite GINA step 4 or 5 treatment [6]. During the management of difficult to treat asthma, certain factors must be evaluated: [40].
The diagnosis of asthma should be confirmed based on the clinical history of the patient and relevant tests before diagnosing difficult to treat asthma [40]. The differential diagnostic tests must be performed to prevent incorrect diagnosis as described in table 2 and 3.
Risk factors that could be modified should be corrected before managing difficult to treat asthma. Modifiable risk factors include incorrect inhaler techniques, smoking (even secondary), poor medication adherence, and environmental factors such as allergens, pollens, or non-specific stimuli.
Co-morbidities must be controlled well before initiating the actual treatment of difficult-to-treat asthma. Common co-morbidities associated with difficult to treat asthma include chronic rhino-sinusitis (CRS), GERD, obstructive sleep apnoea (OSA), obesity, and depression/anxiety disorder. Controlling these co-morbidities can help in the optimization of treatment as concomitant co-morbidity can affect diagnosis, reduce positive therapeutic outcomes, increase acute exacerbation, and result in patients receiving excessive treatment.
Management of patients diagnosed with difficult-to-treat asthma extends beyond asthma pharmacotherapy because multiple patient-related, as well as health status-related factors, need to be addressed before initiating the actual treatment [41].
Patient education is necessary for the management of difficultto-treat asthma. Lack of information about disease, drugs, and lifestyle modifications may lead to poor clinical outcomes. To ensure better disease control, patients with difficult-to-treat asthma and their family need to understand the nature of the disease, signs and symptoms, time of exacerbations, treatment principles, the importance of regular use of asthma medications, avoidance of risk factors or asthma trigger factors, and self-management of asthma [41]. A study revealed that patients who received training on inhalation techniques made significantly fewer errors while using their inhalers (p < 0.0001) [42]. On the other hand, patients who received no instructions were more likely to report frequent exacerbations of asthma than those who received instructions on inhalation techniques by a healthcare provider [43,44].
The use of Inhaled corticosteroids (ICS) with long-acting β2 agonists (LABA) as a combination is a mainstay of asthma treatment. In patients who do not achieve good symptomatic control or optimal clinical outcomes with the use of a medium-dose ICS-LABA combination (GINA step 4), it is recommended that the dose of ICS (a high-dose ICS-LABA combination) must be increased or a second controller agent, such as tiotropium or leukotriene receptor antagonists (LTRA), can also be added. Before moving to GINA step 5 treatment, maintenance and reliever therapy with an Inhaled Corticosteroids (ICS) with a LABA i.e. formoterol combination can be considered. The management of step 4 and step 5 of asthma is described in figure 1 [40].

Figure 1: Management of step 4 and step 5 of asthma.
Patients with uncontrolled asthma despite the use of a maximum dose of Inhaled Corticosteroids (ICS) and long acting beta 2 agonists (LABA) combination and an add-on therapy with another controller agent such as tiotropium, LTRA, or theophylline should be referred for phenotypic assessment by asthma specialists. Not only this, the use of type 2 biologics must be questioned in such patients and determined. Patients with type 2 high inflammation are eligible for type 2 biologics.
The incidence of type 2 inflammation can be seen in 50% of people diagnosed with severe asthma and responds to newer biological agents. It can be identified by increased eosinophil counts in the blood or sputum or elevated ferrous nitrous oxide inhalation. Biological therapy may be considered in patients with type 2 inflammation only if it is available and affordable. If there is no evidence of type 2 inflammation, the evaluation must be done with immunoglobulin E (IgE) testing, skin testing, sputum induction for inflammatory phenotype, bronchoscopy, and high-resolution chest computed tomography to rule out any anatomic obstruction in the respiratory system [45,46].
Inhaled corticosteroids are the mainstay of pharmacological treatment of asthma [45]. According to a study, it is safe to add a Long-Acting Beta-2 Agonists (LABA) to inhaled corticosteroid therapy, although this does not reduce the probability of a serious exacerbation requiring hospitalization [47]. Another study identified that adding a Long-Acting Muscarinic Antagonist (LAMA) to inhaled corticosteroid therapy has superior action over adding a placebo for improving asthma control in patients 12 years or older. But, it was also identified that LAMA add-on therapy was not superior to LABA add-on therapy in terms of its efficacy [48]. The same study also revealed that the addition of a LAMA to therapy in patients already receiving a LABA plus an inhaled corticosteroid (triple therapy) does not further improve asthma control [48]. If Leukotriene Receptor Antagonists (LTRA) and a trial of a high dose of Inhaled Corticosteroid (ICS) were not already used, then it can also be added [45].
Although broad-spectrum antibiotics such as macrolides have been used as an off-label therapy for severe asthma due to their anti-inflammatory and immune-modulating effects, evidence supporting its use in difficult-to-treat asthma especially in children is still conflicting [45,49].
Therapies that target type 2 inflammation pathways include Omalizumab, Mepolizumab, Dupilumab, and Benralizumab. These subcutaneous formulations are very expensive (nearly $1,000 to 3,000 per month) and should be reserved for people with severe asthma. If Mepolizumab, Dupilumab, and Benralizumab are approved for patients of 12 years age and older, then Omalizumab is approved for patients six years and older [50]. A systematic review conducted by the Cochrane database showed that Omalizumab, being an anti-IgE monoclonal antibody, is effective in minimizing asthma exacerbations, hospitalizations, and inhaled corticosteroid dosage in patients with allergic asthma or high serum levels of IgE concentrations and is appropriate only for patients with evidence of allergic asthma on skin testing or elevated IgE [50].
Mepolizumab is also a monoclonal antibody that acts against interleukin-5 (IL-5). In some studies, it has been observed that mepolizumab, an anti-IL-5 drug not only reduces the exacerbations of severe asthma but also improve asthma-related quality of life in patients diagnosed with severe asthma and elevated eosinophil concentrations. In addition to this, Mepolizumab also reduces the need for oral corticosteroids. It is approved as an add-on maintenance therapy for patients diagnosed with severe eosinophilic asthma [51]. Benralizumab mainly targets eosinophils using the IL-5 alpha receptor. In patients with severe uncontrolled asthma, Benralizumab modestly reduces asthma exacerbations and the use of oral corticosteroids. Hence it is approved for severe eosinophilic asthmatics as an add-on maintenance therapy [52]. Dupilumab is a monoclonal antibody that targets the IL-4 alpha receptor and it has been observed that in a study of children and adolescents with moderate to severe uncontrolled asthma, those who were taking Dupilumab therapy had lower rates of severe exacerbations than those with placebo [53].
The dosage information of biologic therapy in the pediatric population is mentioned in the table 4.
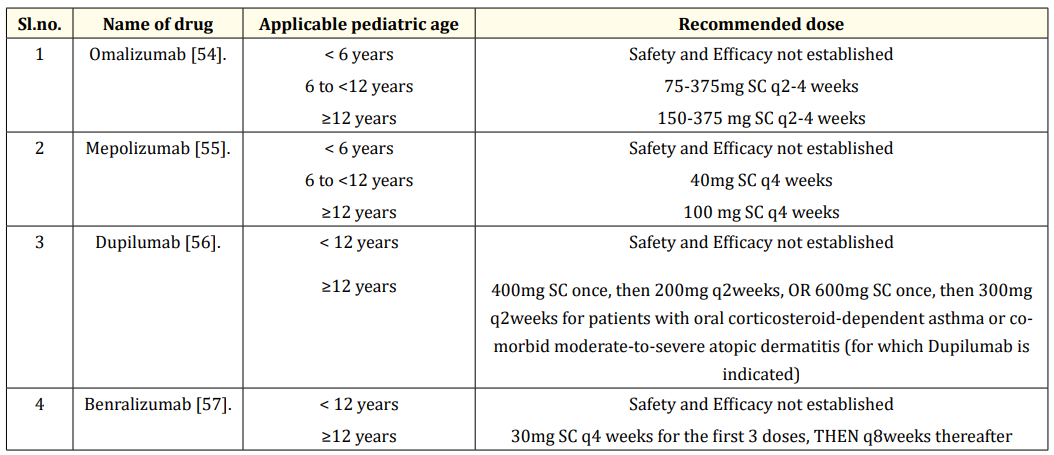
Table 4: The dosage information of biologic therapy in the pediatric population.
Tiotropium is a long-acting muscarinic antagonist (LAMA), which shows its action binding equally well to M1, M2 as well as M3 cholinergic receptors. After binding, it dissociates slowly from the M1 and M3 cholinergic receptors, and hence the long duration of the bronchodilator effect can be seen in patients who use tiotropium. It can be given once daily (2.5mcg), maintenance treatment of asthma in pediatric patients aged above 6 years as it has a long duration of action due to which it lasts for 35 h with maximum effect (Maximum pharmacodynamic action observed at peak concentration of drug in blood i.e. Cmax) within 60 min. Thereby by it helps in improving compliance in children [58,59].
Recently five pediatric randomized controlled trial studies regarding the use of Tiotropium in the pediatric population have shown promising results [60-65]. Tiotropium delivered via an inhaler called Respimat® Soft Mist™ inhaler has recently been approved in the USA in February 2017 for use as once-daily maintenance therapy for children with asthma over the age of 6 years old. In addition to this, Tiotropium is also recommended by GINA guidelines as an add-on therapy option at Steps 4 and 5 of asthma with a history of acute exacerbations, in patients aged 12 years and above. A study from a large clinical trial program comprising a wide spectrum of asthma severity in children and adolescents has demonstrated that Tiotropium Respimat® as an add-on therapy to inhaled corticosteroids (ICS), is not the only well-tolerated but also have efficacious bronchodilator action, resulting in improved lung function [60]. This result is also reflected in several other studies including many clinical trials which have shown improved lung function where tiotropium Respimat™ is used as an add-on therapy to ICS (Inhaled corticosteroids) in patients with poorly controlled asthma [65,66].
A study conducted by Bisgaard H and team showed that the incidences, as well as the patterns of adverse events (AE), were similar between Tiotropium Respimat® 5 mcg, 2.5 mcg, and placebo therapy, as an add-on treatment to inhaled corticosteroid with or without a secondary controlling agent, in pediatric patients aged 6-17 years experiencing symptomatic asthma [66]. Another randomized, double-blind, placebo-controlled trial study conducted by Elianne J L EVrijlandt and colleagues revealed that tolerability of Tiotropium in the pediatric population aged between 1-5 years was similar to that of a placebo, which is similar to previous findings in older populations. Although mean daytime asthma symptom scores were not significantly different between 3 groups (i.e. 2.5mcg, 5mcg, and placebo), tiotropium showed the potential to reduce the risk of asthma exacerbation in children of 1-5 years old compared with placebo [67].
A few medical regimens (nonstandard therapy) have been shown to have some clinical benefit in the treatment of refractory asthma. The use of a single dose of intramuscular triamcinolone for difficult pediatric asthmatics has been shown to reduce the inflammation and the frequency of asthma exacerbations, respectively [68]. The reasons for its effectiveness may be a combination of improved compliance, improved anti-inflammatory profile of parenteral steroids, and overcoming a relative resistance to steroids. Lastly, Immuno-modulating Agents such as the deoxyribonucleic acid (DNA) vaccine in both preclinical and early clinical stages hold promise for high therapeutic potential and may become future options for pediatric patients diagnosed with difficult-to-treat asthma [69].
Pediatric population with difficult-to-treat asthma is the cause of concern because despite maximal conventional treatment these patients experience frequent exacerbations of asthma. They pose a major challenge to healthcare professionals and family members because of the adverse effects of high doses of corticosteroids, impaired quality of life, continued reduction in lung function, and increased healthcare costs. The living hood of children with difficultto-treat asthma is severely disrupted with unscheduled visits and emergency care and hospital admissions [70]. Thus management of these patients extends beyond asthma pharmacotherapy because multiple other patient-related domains need to be addressed as well. Electronic monitoring and medication adherence must be considered and measures must be taken to prevent non-compliance. The study involved a brief review on assessment of risk factors in children with difficult to treat asthma and pharmacological management that included optimal use of long-acting muscarinic antagonist (Tiotropium) and biological monoclonal antibody treatment (Omalizumab, Mepolizumab, Dupilumab, or Benralizumab) use of which is relatively new in pediatrics. Hence, individualized treatment must be preferred in these patients for optimal clinical outcomes.
None.
Copyright: © 2021 Edwin Dias and Shwetha. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.