Foli Yvon Agbeko1,2*, Mawouto Fiawoo1,3, Magnoulelen Nzonou4, Mawaba Peleke Hilim5, Bawoubadi Wangala6, Manani Hemou2, Fidèle Comlan Dossou6, Sollim Talboussouma7, Rachel Bayahou Kérékou2, Homba Daké Batalia3, Rollin Arnaud Djomaleu3, Kokouvi Evenyo Abalo3, Mazama Pakoudjaré2, Edem Koffi Djadou1,3 and Komi Assogba8
1Département de Pédiatrie, Faculté des Sciences de la Santé, Université de Lomé, Togo
2Service de Pédiatrie, Centre Hospitalier Universitaire Campus, Lomé, Togo
3Service de Pédiatrie, CHU Sylvanus Olympio, Lomé, Togo
4Service de Pédiatrie, Hôpital de Bè, Lomé, Togo
5Direction Préfectoral de la Santé de Sotouboua, Sotouboua, Togo
6Centre Hospitalier Régional de Sokodé, Sokodé, Togo
7Service de Pédiatrie, CHU Kara, Kara, Togo
8Département de Neurologie, Faculté des Sciences de la Santé, Université de Lomé, Togo
*Corresponding Author: Foli Yvon Agbéko, Département de Pédiatrie, Faculté des Sciences de la Santé, Université de Lomé, Togo.
Received: March 15, 2021 ; Published: April 19, 2021
Citation: Foli Yvon Agbeko., et al. “Re-Emergence of Meningococcal Meningitis W135 in Togo: Description of the Epidemic in Children ”. Acta Scientific Paediatrics 4.5 (2021): 24-28.
Background: Epidemics of meningococcal meningitis are common in several african countries, including Togo. In northern Togo, part of the “meningitis belt”, the A and C serogroups was the primary causal agents. The objective of this work was to estimate frequency of acute bacterial meningitis (ABM) in childhood in hospital admissions during the 2016 epidemic in Togo, to describe clinical and bacteriological features, and to measure the extent of meningococcal serogroup W135 among children.
Methods: This is a cross-sectional, multi-centre study, carried out among children aged 0 to 15 years, hospitalised for ABM between 1st January and 31st March 2016 in the four district hospitals of the Central Region in Togo. The operational case definitions are taken from the WHO reference document.
Results: The overall hospital frequency among children was 4.80% for suspected meningitis case, 2.86% for probable cases and 1.55% for confirmed cases. Children (0 - 15 years) accounted for 63.60% of patients hospitalised for ABM. The M/F sex ratio of children was 1.42 and the mean age was 6.51 years (7 days - 15 years) with 59.56% (81/136) beyond to 5 years. The hospitalisations occurred in February-March (97.1%). Chief presentation was high fever (77.20%) and headache (39.97%). Meningeal signs were present in 51.97%. Apart from the negative results (59.26%) on direct examination of the cerebrospinal fluid (CSF), meningococcus was the confirmed germ in 90.91%. The serogroup W135 was isolated in 55.00% and the serogroup A in 32.50%. The overall case-fatality rate was 9.55%.
Conclusion: Meningococcus W135 was the agent responsible for the 2016 epidemic in Togo. Children were mostly affected. The tetravalent meningococcal A+C+Y+W135 vaccination should be instituted in Northern Togo, primarily in children under 15 years of age.
Keywords: Meningitis; Neisseria meningitidis; W135; Children; Togo
Five hundred thousand cases of meningococcal infections are reported annually by 2018, half of them in sub-Saharan Africa [1]. Explosive epidemics are occurring in Africa, with annual incidence rates of up to 800 per 100,000 per country [2]. Thirteen serogroups of Neisseria meningitidis (Nm) have been described, the most common of which are the serogroups: A, B, C, W135, X and Y; the others are more rarely isolated [3]. The A and C serogroups was the primary causal agents. The frequent mass vaccination campaigns against meningococcus A, and sometimes C in Africa, seem to be the basis for the emergence and spread of serogroup W135 [4, 5]. In northern Togo, part of the “meningitis belt”, the lethality is high. Togo has experienced several acute bacterial meningitis (ABM) epidemics in 1997, 2004, 2007, 2014 and 2016, dominated by serogroups A and C [6].
The objective of this work was to estimate frequency of ABM in childhood in hospital admissions during the 2016 epidemic in Togo, to describe clinical and bacteriological features, and to measure the extent of meningococcal serogroup W135 among children.
The Central Region is located in the heart of Togo, with a surface area of 13,307 km2 and a population estimated in 2019 at 742,661 inhabitants [7]. This region comprises four health districts (Tchaoudjo, Tchamba, Sotouboua and Blitta) each with a main hospital, respectively: the Regional Hospital Centre of Sokodé (CHR Sokodé), the district Hospital of Tchamba (CHP Tchamba), the Prefectoral Hospital Centre of Sotouboua (CHP Sotouboua) and the Prefectoral Hospital Centre of Blitta (CHP Blitta) (Figure 1). The capital of the Central region, the town of Sokodé, is home to the regional referral hospital (CHR Sokodé) [7,8]. This multi-centre cross-sectional study was conducted in the paediatric/medical wards of the four districts hospitals. It focused on the files of children aged 0 to 15 years who were hospitalised. The study ran from 1st January and 31st March 2016. Operational definitions were taken from the WHO guidelines “Standard operating procedures for surveillance of meningitis preparedness and response to epidemics in Africa” (Table 1) [9].
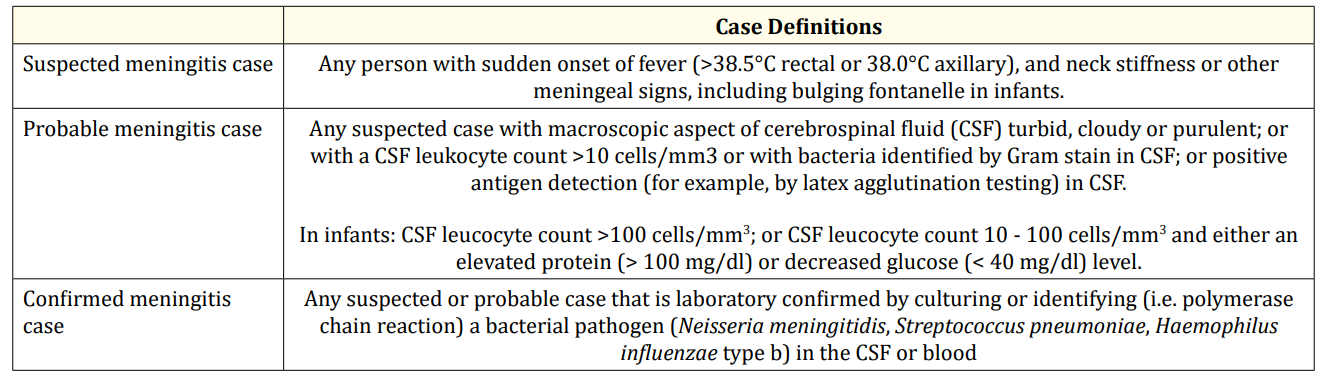
Table 1: Case definitions for acute bacterial meningitis according to WHO (2019).
![Figure 1: Health mapping of Togo [DRS Centrale/MSPS].](https://actascientific.com/ASPE/images/IJMCR/ASPE-04-0395-fig1.PNG)
Figure 1: Health mapping of Togo [DRS Centrale/MSPS].
Not all cases of non-bacterial meningitis and cases with incomplete records were included. The parameters studied were hospital frequency, sociodemographic parameters (age, sex, seasonality), clinical data (convulsions, coma, vomiting, infectious syndrome, meningeal syndrome), biological data (CSF macroscopy, cytology, direct examination, research of soluble antigens, culture, latex at CHR Sokodé), outcomes data (recovery or death, length of hospitalisation). The four laboratories have direct examination for the identification of meningitis germs. The treatment recommended and available in the pharmacy was ceftriaxone and cefotaxime. In addition to direct examination, the laboratory of CHR Sokodé and CHP Sotouboua have cytological and biochemical examinations for the identification of meningitis germs and latex kits for the determination of serogroups. For the identification of the germ, in general we proceeded as follows [9]: i) Observation of the colonies that have appeared, search for catalase, oxidation reaction. ii) Microscopic examination after gram staining iii) Latex reagglutination. The biochemical analysis of the CSF had not been carried out. The investigators consisted of the epidemiological surveillance focal points of the four health districts, supervised by laboratory managers and district medical officers. Data processing was done using SPSS version 20 software. The figures and tables were designed using Microsoft Excel version 2013. A study authorisation was obtained from the Regional Health Directorate (central region) and anonymity respected for each file studied.
The number of hospitalizations was 2,830 in the four district hospitals of the Central Region during the period of the study. Two hundred and fourteen adults and children were suspected of ABM. Children under age 15 accounted for 63.55% (136/214) of suspected cases. The overall hospital frequency among children was 4.80% for suspected meningitis case, 2.86% for probable cases and 1.55% for confirmed cases.
The mean age was 6.51 years (17 days - 15 years). Children aged beyond 5 years accounted for 59.56% (81/136) and male for 58.82% (sex ratio M/F 1.42) (Table 2).
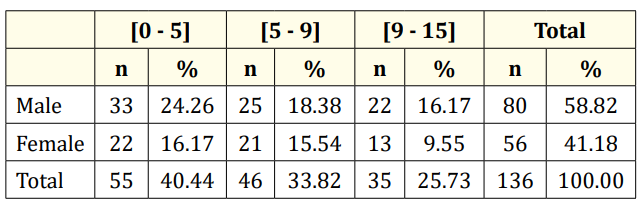
Table 2: Distribution of ABM cases by age group and gender.
Eighty-four patients (61.76%) resided in rural areas and 48.53% (n = 66) in Sotouboua district. The months of February (n = 73; 53.67%) and March (n= 59; 43.38%) were the most morbid months. No patient had received a meningococcal vaccine before the hospitalisation.
Chief presentation was high fever (77.20%), headache (39.97%), convulsions (21.32%), vomiting (19.11%) and coma (16.17%). An infectious syndrome was objectively found in 77.20%. Meningeal signs were present in 51.97%. The most common meningeal signs were stiffness of the neck (43.38%), then Kernig/Brudzinski’s signs (21.32%). The bulging of the fontanelles was reported in 1.47% of cases.
The macroscopic aspect of the CSF was pathological, revealing ABM in 75.00% (81/108) cases (probable cases).
Table 3 shows the results of the macroscopic examination of the CSF by hospital.
Direct examination of the CSF by Gram stain was negative in 64 out of 108 cases (59.26%). For the positive direct examinations (n = 44), Neisseria meningitidis (90.91%) and Streptococcus pneumoniae (9,09%) were visualized.
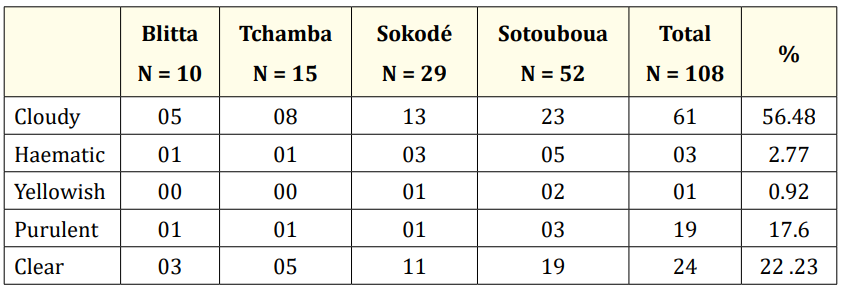
Table 3: Results of the macroscopic examination of the CSF by hospital (N = 108).
In the cytological study of the CSF (n = 29), more than half of the samples (51.71%) had exceeded the threshold of 100 elements/ mm3 : 0 - 10 elements/mm3 (37.97%), 10 - 100 elements/mm3 (10.34%), 100 - 500 elements/mm3 (6.89%) and significant pleocytosis (500 elements/mm3 ) in 44.82%.
CSF culture was carried out only on 05 cases in Sotouboua and was positive in 40% of the cases (Neisseria meningitidis 100%).
The search for soluble antigens for the different serogroups of meningococcus (n = 40) revealed NmW135 (n = 22; 55.00%) and NmA (n = 13; 32.50%). For 05 samples, the serogroup could not be identified.
Mono antibiotic therapy based on 3rd generation cephalosporins (cefotaxime or ceftriaxone) has been used in all children. Thirteen cases of death (case fatality rate of 9.55%) were observed. The average length of hospitalisation was 7 days (0 - 12 days).
The overall hospital frequency of ABM in children was 4.80%. Lower incidences have been reported in other African countries, notably in Gabon in 2000 (1.2%) [10], Tunisia in 2006 (1.14%) [11] and Senegal in 2016 (3.45%) [12]. This can be explained on the one hand by the fact that Gabon and Tunisia are not part of the Meningitis belt and on the other hand Senegal is a Sahelian country which is part of this belt.
The preferred age group was pre-adolescents and adolescents (59.56%) as confirmed in Burkina Faso in 2014 [13] and in Guinea in 2013 [14]. On the other hand, Ba in Senegal in 2016 returned to a predominance of under-fives (53 months) due to different inclusion criteria. Male over-morbidity is generally reported [12,13]. The high cost of the tetravalent meningococcal vaccine (60 USD), the non-availability of the trivalent vaccine and ignorance of its existence explain the status of children in these rural areas. All cases in our study occurred during the dry period (November to March), with a peak in frequency in February and March.
This period of the dry season in Togo corresponds to the harmattan, which maintains microorganisms [12,17,18]. The clinical signs was suggestive of meningitis in all cases. The signs were dominated by fever and a meningeal syndrome (bulging fontanelle, stiff neck, Kernig’s and Brudzinski’s sign). The meningeal syndrome was frank, considering the age group most affected in our study (5 to 15 years) [13].
The macroscopic appearance of the CSF is very suggestive of bacterial meningitis, the CSF is cloudy or purulent in the majority of cases [19,20]. However, a clear CSF does not rule out the diagnosis, as it may be meningitis that has been beheaded by antibiotic therapy or at the beginning [21,22]. One hundred and eight children had received spinal puncture with a cytobacteriological study of the CSF. In more than half of the cases (59.26%), the germ involved could not be identified. This can be explained by the abusive use of antibiotics at home in the face of any infectious syndrome in children. Pleiocytosis is an important element in the diagnosis of paediatric bacterial meningitis. Significant pleocytosis (˃ 500 elements/mm3) was present in 44.82%. A significant cellular reaction in the CSF is often related to late diagnosis [19]. Neisseria meningitidis was the causative agent in 41.17% of cases in the central region in 2016. Streptococcus pneumoniae was rarely isolated (n = 04) [23,24] and Haemophilus influenzae b was never found. In subSaharan Africa, particularly in the meningitis belt, the serogroup A was still predominant. Indeed, between 1995 and 2000, Camara in Senegal had found serogroup A in 86% [15]. During the 2004 epidemic season in Togo, NmA accounted for 83% of Nm cases [6]. Nevertheless, in our study, serogroup W135 was the most common (55.00%). This can be explained by the fact that an epidemiological transition has been observed in some countries of the Lapeysonnie belt since 2002 with the emergence of serogroup W135 [25,26]. This followed the global epidemic outbreak in 2000 that started in Saudi Arabia caused by NmW135 [25]. After the return of pilgrims from Mecca in mid-March 2000, the first Togolese cases of meningococcal meningitis W135 appeared in 2004; 207 cases were included in Togo (143 confirmed), Nm 46%, Sp 36% and Hib 17%. NmW accounted for 8% of cases [6]. In addition, frequent vaccination campaigns against meningococcal A could encourage the emergence of NmW135 [27].
In general, the clinical course was favourable on 3rd generation cephalosporins (C3G) for 7-10 days in the absence of an antibiogram [28]. The case fatality rate was 9.55%. Higher mortality was reported in Burkina Faso in 2006 (15.5%) [27] and Guinea in 2013 (13.8%) [14]. The data were lower in Senegal in 2003, in 2016 (respectively 3.8% and 4.5%) [12,15] and in France in 2012 (6.5%) [16]. The average duration of treatment was 7 days in our study.
The medium and long-term medical follow-up of the children, which would allow the detection of possible neurological sequelae, could not be carried out due to lack of funding and the multicentric nature of the study.
This multicentre study of 136 cases of ABM in hospitalized children under 15 years of age showed over-morbidity in boys aged 5 to 15 years. The meningeal syndrome was suggestive.
Antibiotic therapy was most often favourable, despite a case fatality rate of 9.55%. It would be beneficial to introduce the trivalent A+C+W135 or tetravalent A+C+Y+W135 vaccine in children under 15 years of age in northern Togo.
Copyright: © 2021 Foli Yvon Agbeko., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.