Sarah Falou1#*, Ghina Kahil2#, Batoul Kawtharany1, Samar Dalle2, Zahraa Slim1, Ali Jibbawi1, Imad Chokr1, Nawfal Nawfal1, Lea Chokr1 and Rabab El Hajj1
1Department of Pediatrics, Faculty of Medical Sciences, Lebanese University, Lebanon
2Depatment of Pediatrics, Faculty of Medical Sciences, Beirut Arab University, Lebanon
#Contributed Equally to this work
*Corresponding Author: Sarah Falou, Department of Pediatrics, Faculty of Medical Sciences, Lebanese University, Beirut, Lebanon.
Received: February 27, 2021; Published: March 11, 2021
Citation: Sarah Falou., et al. “PICU Admission in an Asthmatic COVID Positive Child: Case Report”. Acta Scientific Paediatrics 4.4 (2021): 30-35.
The current pandemic of COVID 19 infection globally has been associated with a variety of pediatric presentations. Children have been relatively spared from severe COVID-19-related illness. Early reports suggest that most children infected with severe acute respiratory syndrome coronavirus 2 (“SARS-CoV-2”) are usually asymptomatic, or have mild symptoms, and do not require hospitalization. What is not known is whether children with chronic respiratory illnesses have exacerbations associated with SARSCoV-2 virus. We present an asthmatic 11-year old boy with cough, fever, and dyspnea. Reverse transcription (RT) polymerase chain reaction (PCR) confirmed COVID-19 on a nasopharyngeal sample. Ct scan chest revealed multiple bilateral diffuse ground-glass infiltrates severe in extension suggestive of COVID-19 pneumonia. His hospital stay was complicated with superinfection and severe respiratory distress that necessitate transfer to PICU with requirement of high flow nasal cannula with non-rebreather face mask in addition to asthma treatment. COVID-19 may present with mild pneumonia in children or with an exacerbation of asthma in asthmatic children. Based on an extensive search of the literature to identify children with asthma who have contracted SARS CoV-2 infection, there were a very limited number of pediatric studies that reported specific pediatric asthma and COVID-19 data.
Keywords: COVID-19; Asthma; Pneumonia; Respiratory Distress; High Frequency Nasal Canula
The novel coronavirus COVID-19, first identified in December 2019 in China, is currently causing a global pandemic. SARS-CoV-2 seem to less commonly affect children and to cause less severe disease compared with adults. Preliminary evidence suggests that children are as likely as adult to become infected with SARSCoV-2 but are less likely to be symptomatic or to develop severe symptoms. Most pediatric cases present with mild symptoms like cough and fever, even so some children may develop severe illness especially those with underlying medical conditions [1]. Alternatively, asthma, the most common chronic respiratory disease of childhood, is a leading cause of emergency department visits, and 1 of the top 3 indications for hospitalization in children. Asthma is a chronic inflammatory disease of the airways, characterized by recurrent episodes of airflow obstruction that results from edema, bronchospasm, and increased mucus production. In most cases of asthma in children, multiple triggers have been recognized, of which we can mention respiratory infections, allergens, irritants, and other environmental factors.
Classic symptoms of asthma include cough, wheezing, chest tightness, and shortness of breath. Children may also experience varying degrees of tachypnea and dyspnea. Symptoms are often episodic and may be worse at night. Despite advances in the management of pediatric asthma, significant disparities in care and outcomes persist [2].
An 11-year-old male, with a history of well-controlled asthma, presented complaining of dry cough and high grade fever that started few days prior to presentation. History goes back to 4 days ago when the patient started to experience high grade fever reaching 39 degrees Celsius partially responding to antipyretics associated with a dry cough. Patient sought medical advice when chest x ray done showed bilateral basal infiltrates (Figure 1) and started on albuterol nebulizer, dexamethasone, and amoxicillin-clavulanic acid for asthma exacerbation due to pneumonia. However, the patient’s symptoms had worsened with increased work of breathing and tachypnea, thus he was rushed to the emergency department.

Figure 1: Chest xray showed bilateral basal infiltrates.
Upon arrival patient was in respiratory distress with SPO2 of 67% and decreased bilateral air entry with diffuse crackles and wheezes upon auscultation. Heart rate, blood pressure and temperature were within normal limits. Patient was stabilized and put on 5 L/min oxygen supply by face mask, after which the saturation increased to 96%. He received inhaled short acting beta-2 agonists and ipratropium bromide for 3 consecutive doses with IV methylprednisolone for moderate-severe asthma exacerbation. CT Scan was done and showed multiple bilateral diffuse ground- glass infiltrates more in the upper lobes and severe in extension (Figure 2a2c). PCR COVID-19 was taken prior to admission, and the patient was admitted to regular isolation pediatrics floor. To note that the mother denied any history of sick contacts.

Figure 2a

Figure 2b

Figure 2c
Upon admission, full workup was done and showed leukocytosis with neutrophilia and elevated inflammatory markers (Results showed in table 1). Electrolytes and liver function test were within normal range. He was started on ceftriaxone and clarithromycin for suspected bacterial pneumonia; as well as asthma exacerbation management that included intravenous dexamethasone, ipratropium bromide, albuterol and budesonide puffs (nebulizers were avoided for suspicion of COVID-19). Soon after admission, PCR COVID-19 turned out to be positive, so antibiotics were stopped and supportive management was initiated. This included IV hydration, antipyretics, Zinc, vitamin D, and vitamin C. At that time of hospitalization, the patient was on oxygen supplementation reaching a maximum of 10 L/min by face mask. On the 8th day of hospitalization, the patient experienced severe tachypnea with subcostal and suprasternal retractions and desaturation reaching 60%. Adding to these tachycardia and chest discomfort. Cardiac enzymes were taken and were within the normal range (CPKMB: 0.991, CPK: 39, troponin: 0.005), ECG was done with no abnormal findings, so cardiac involvement with COVID was excluded. CT angiography result came negative for pulmonary embolism. There was an increase of CRP reaching up to 103.5 mg/dl, raising the suspicion of superimposed bacterial infection. The patient was then started on meropenem, vancomycin, and fluconazole for broader coverage; in addition to enoxaparin sodium. At that time patient was put on double source oxygen supply by nasal cannula and non-rebreather face mask, providing flush oxygen by both sources with no clinical improvement. Decision to transfer the patient to PICU was taken. Upon arrival to PICU, patient was put on High flow nasal cannula HFNC (flow 25 and Fio2 100%) in addition to non-rebreather face mask. Saturation improved to 93% and tachypnea decreased. Two days later, patient improved clinically with a saturation of 98% and normal respiratory rate, parameters were decreased gradually until removing HFNC after four days of admission to PICU. Blood culture showed no growth. Patient was then transferred to regular isolation pediatrics floor to continue the antibiotic course, during which weaning of oxygen supply was done. On day 14 of hospitalization, patient was completely off oxygen with 99% saturation. PCR COVID was repeated and came out negative, and patient was discharged home (repeated labs before discharge showed in table 1).
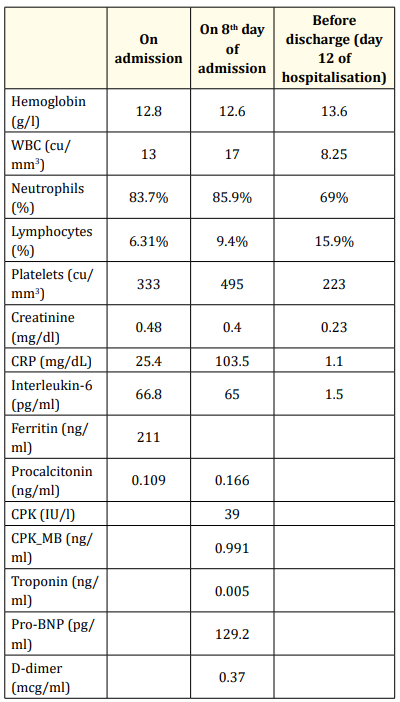
Table 1: Showing laboratory investigations done during hospital stay.
The current pandemic of SARS-CoV-2 infection globally has been associated with a variety of pediatric presentations depending on the stage of the disease, the prior health status, and the presence of comorbidities and other individual features, with more frequent asymptomatic infection in children than in adults [3]. Children may also present with mild pneumonia, Dong., et al. reported that 90% had mild to moderate form of the disease [4]. A metanalysis done by Assaker R., et al. also demonstrated that 16% of children were asymptomatic and 82% of them had mild to moderate infection [5]. However a review done in Wuhan Children’s Hospital showed that 3 out of 1391 children required intensive care support and invasive mechanical ventilation but all had coexisting conditions (hydronephrosis, leukemia, and intussusception) [6].
In a retrospective study done in New York done by Zachariah P., et al. [7] showed that obesity was significantly associated with disease severity but asthma was not statistically significant factor for the disease severity. However, as our patient had a normal BMI of 20.5 kg/m2. This study also showed that patients who presented with shortness of breath tend to be more sick, with elevated inflammatory markers in those with severe disease [7].
Given the lack of pediatric asthma and COVID-19 cases in the literature, and based on a systematic review done by Castro-Rodriguez and Forno [8] there were a very limited number of pediatric studies that reported specific pediatric asthma and COVID 19 data. Moreover, only two reports in children included information on asthma as a potential risk factor for COVID-19 infection, but not for severity or mortality [8].
To our knowledge, 2 cases in the literature were reported, the first by Barsoum Z. who reported a case of an asthmatic 12-year old girl that presented with cough, wheeze, and mild pneumonia due to COVID-19, she was discharged home 2 days following hospitalization [9].
On the other hand, the second case done by Aghdam MK., et al. who reported the case of a child with asthma who sought care for COVID-19 symptoms and whose condition did not improve despite appropriate treatment for asthma, pneumonia and COVID-19. Further examination revealed foreign body aspiration which has been removed by bronchoscopy [10].
We report the successful management of an 11-year-old asthmatic child with COVID-19 in Lebanon who require PICU admission. Our patient, with underlying well controlled asthma presented with fever, cough, tachypnea, shortness of breath and desaturation diagnosed as moderate to severe asthma exacerbation according to Pediatric Respiratory Assessment Measure (PRAM) [11] due to severe pneumonia secondary to COVID-19, and managed by intravenous dexamethasone, ipratropium bromide, albuterol and budesonide puffs, high flow nasal canula and non-rebreather face mask to maintain saturation above 93%. IV antibiotics was also used to treat the suspected superimposed bacterial infection. The patient was started on prophylactic anticoagulation, knowing that anticoagulation therapy was recommended according to an article discussing the benefits of such treatment in children with COVID-19 [12]. Conversely, a survey done in 174 centers, COVID-19 in children with underlying chronic respiratory diseases showed that infection with SARS-CoV2 in patients having asthma and cystic fibrosis was well tolerated, but a substantial minority of children with BPD and other conditions required ventilatory support indicating that these latter groups are at risk from SARS-CoV-2 infection [13].
Though, it also remains unclear if COVID-19 increases the risk of asthma exacerbations and if covid-19 could trigger a viral induced exacerbation of asthma in asthmatic children as showed by a serial of case series conducted by Abrams E and Szefler S [14]. Our case demonstrated that COVID19 may present with a clinical picture of asthma exacerbation in asthmatic children.
The Global Initiative for Asthma (GINA) on March 2020 recommends avoiding the use of nebulizers due to the increased risk of disseminating COVID-19 to other patients and health care staff; they thus recommend the use of pressurized metered dose inhalers (pMDI) as the preferred delivery system during asthma attacks [15]; therefore, our case was managed with inhaled Short acting beta-2 agonist, ipratropium bromide and budesonide.
GINA [15] and the British Thoracic Society [16] do not recommend avoiding corticosteroids for acute asthma attacks even if due to COVID-19. Hence our patient was managed by IV corticosteroids for its moderate to severe asthma exacerbation.
However further data is needed to identify whether childhood asthma (or other pediatric respiratory diseases) are associated with COVID-19 risk or severity [8] and whether there is an increased risk of COVID-19 morbidity among children with asthma [14].
We suggest that the reported case supports assuming at the conclusion: The case related suggests that COVID-19 may increase the severity of asthma in children. Differentiating acute COVID-19 infection from asthma exacerbation, is challenging especially in pediatric age group. Therefore, further studies and collaborative international efforts are required to identify the impact of pediatric asthma on the course of SARS-COV-2 infection and to determine whether asthma in children is a potential risk factor for COVID-19 mortality and morbidity. As a result, families and pediatricians have an essential role in ensuring that asthmatic children maintain good asthma control during this global pandemic.
Copyright: © 2021 Sarah Falou., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.