Shagun Singh1 and Roosy Aulakh2*
1Senior Resident, Department of Pediatrics, Government Medical College and Hospital, Chandigarh, India
2Professor, Department of Pediatrics, Government Medical College and Hospital, Chandigarh, India
*Corresponding Author: Roosy Aulakh, Professor, Department of Pediatrics, Government Medical College and Hospital, Chandigarh, India.
Received: January 27, 2021; Published: February 22, 2021
Citation: Shagun Singh and Roosy Aulakh. “Vitamin D Deficiency in Children: Do We Know it All?”. Acta Scientific Paediatrics 4.3 (2021): 64-69.
Vitamin D is an essential micronutrient in maintenance of bone health in children. Deficiency of vitamin D is being increasingly reported amongst all age groups. Over the last few years, various studies have been undertaken on vitamin D status, it’s impact on health and disease and benefits of supplementation in therapeutic doses [1]. Various regimens have been described for management which include daily dosing and also single large dose (stoss therapy) administration. Compliance with daily therapy can be difficult, making high-dose, short-term vitamin D (stoss) therapy attractive to correct vitamin D deficiency. In this review we shall elaborate the existing recommendations on definitions of deficiency, prevention and treatment of vitamin D deficiency in children.
Literature Review: This review has been compiled after extensive literature search using Pubmed and Google Scholar. References of the retrieved articles were further used to expand the research. Also, hand search was done from standard pediatric textbooks and Indian Journal of Pediatrics.
Keywords: Vitamin D; Children; Cholecalciferol
Vitamin D has a vital role in maintenance of bone health via regulation of calcium homeostasis in the body. All parts of the world, including India have increasingly reported vitamin D deficiency amongst various age groups [2,3]. Vitamin D exists in two forms, D3 (cholecalciferol) which is derived from animal sources and ergocalciferol (D2), which is derived from plants. In humans, vitamin D synthesis occurs in the skin on exposure to sunlight. Combination of a habitually low intake with an indoor lifestyle and sun avoiding behaviour has perpetuated an epidemic of vitamin D deficiency. Deficiency of Vitamin D can result in rickets or osteomalacia in an younger or older child respectively [4]. Vitamin D deficiency also negatively impacts peak bone mass causing low bone mineral density in childhood, which can subsequently lead to osteoporosis in adulthood. Maternal vitamin D deficiency may result in hypocalcaemia seizures and at times, cardiomyopathy in neonates [5]. Vitamin D is also being increasingly implicated in the pathogenesis of extra skeletal manifestations including various autoimmune conditions like asthma and infectious conditions.
Given the vast literature on vitamin D deficiency, different recommendations by various organizations, lack of consensus on the ranges for sufficiency and, the pediatrician may be confused regarding the appropriate regimen for prevention and management of vitamin D deficiency [6]. The Indian Academy of Pediatrics recommends a daily dose of 400 IU/day for neonates, 1000 IU /day for infants and 3000 - 6000 IU/day for children between 1 - 18 year. For some patients, adherence to a daily dosing regimen may be difficult, in which case stoss therapy has been recommended [7]. The data on efficacy, safety and effective dosing of stoss therapy is limited. With this article, we have reviewed multiple guidelines comparing daily regimen with stoss therapy.
Vitamin D is obtained from exogenous sources as well as synthesised endogenously. More than 90% of the primary form (cholecalciferol) is synthesised in the skin from direct exposure to sunlight [8]. The remaining needs are met from dietary sources in either form, vitamin D3 or vitamin D2 (ergocalciferol). Both, D2 and D3 first undergo hydroxylation in the liver to form vitamin D, 25-hydroxy vitamin D (25[OH]-D, Calcidiol, or calcifediol) [8]. Subsequent hydroxylation occurs in the kidneys, resulting in formation of active form, 1,25-dihydroxyvitamin D. Various factors like PTH, serum phosphate levels and growth hormone levels influence calcitriol synthesis.
The prevalence of vitamin D deficiency is reported as 50 - 90% in the Indian subcontinent [9]. Low dietary calcium, darker skin colour and changing lifestyle have been regarded as influencing factors. Breast fed infants are at particular risk if mothers are deficient. Various risk factors that predispose to vitamin D deficiency have listed in table 1. Apart from deficient intake, there are multiple causes of non-nutritional rickets, which are not being discussed in detail in this article [10].
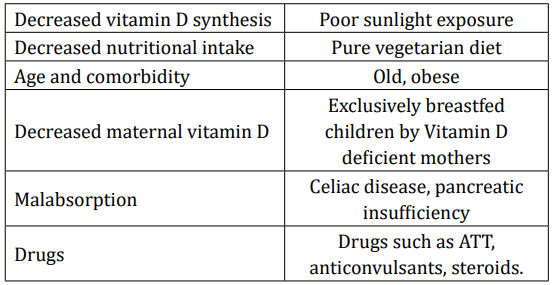
Table 1: Common causes of vitamin D deficiency.
Vitamin D deficiency can lead to a plethora of manifestations amongst infants and children. Clinical features include bony pain and tenderness [11]. Characteristic skeletal deformities of rickets and easy bone fractures can result in growth failure and short stature. Features of hypocalcemia (tetany, spasms, seizures, cardiomyopathy) can also occur in severely deficient individuals [12].
Vitamin D deficiency has also been implicated as a novel risk factor in the pathogenesis of cardiovascular disease, metabolic syndrome and hypertension [13]. Various studies have come up describing its role in causation of Type I as well as Type II diabetes. Active form of Vitamin D has also shown to possess antimycobacterial properties and has also shown to induce autophagy in HIV, in vitro.
The cut off for serum concentration of vitamin D that constitutes is debatable and not well supported by clinical trials, especially in the pediatric population [14,15]. Various societies have given definitions of deficiency, insufficiency and sufficiency of vitamin D levels. According to most studies, levels above 20 have been found to be adequate to prevent complications of deficiency amongst children, covering the needs of 97.5 percent of the population. Table 2 summarizes the definitions given by different organizations [16]. According to the India Academy of Pediatrics, Vitamin D levels < 12 ng/ml are defined as deficient, 12 - 20 ng/ml as insufficient and > 20 ng/ml as sufficient.

Table 2: Definitions of vitamin D deficiency. KDOQI: Kidney Disease Outcomes Quality Initiative; ESPHAN: European Society for Paediatric Gastroenterology, Hepatology and Nutrition.
The major circulating form of vitamin D is 25(OH)D. It has a halflife of 2-3 weeks, compared with 1,25 (OH)2D (calcitriol) which is the active form, and has a half-life of only 4 hours [17]. The active form is influenced by elevated levels of PTH which induce 1- alpha hydroxylase enzyme in the kidney and subsequent normalisation of 1,25 (OH)2 D. Also, the serum concentration of calcitriol is 1001000-fold lower than 25(OH)D, making it an unsuitable indicator of body stores of vitamin D [18].
Various techniques have been used to measure the levels of 25(OH) vitamin D. HPLC (high performance liquid chromatography and TMS tandem mass spectrometry have been reported as gold standard [17]. Radio-immune assays using monoclonal antibodies to 25(OH)D and chemiluminescent protein binding assays include other methods.
IAP doesn’t recommend routine screening of healthy children for vitamin D deficiency. Screening may however be performed for high-risk groups. At risk groups include children with history of rickets, fat malabsorption, liver or renal insufficiency, those on anti-epileptic drugs, transplant recipients, children on treatment for malignancy, children with predisposition to osteoporosis such as in hypogonadism or Cushing’s syndrome. The need for blood sampling, high cost of assessment and low positive predictive value, preclude mass screening for vitamin D deficiency [19].
For the treatment of vitamin D deficiency rickets, the IAP recommends an initial 2- to 3-month of 400 IU in neonates, 2000 IU daily in infants 1 to 12 months old, and 3000 - 6000 IU daily in patients over 12 months old [20] (Table 3). Although radiologic evidence of healing occurs within 2 to 4 weeks of treatment, large dose treatment (of either vitamin D3 or D2) should be continued for 2 to 3 months. A maintenance dose of 400 IU is recommended in all age groups, after sufficient calcidiol concentrations are achieved. Recommendations by AAP, the Endocrine Society and Institute of Medicine are summarised in table 4 and 5 respectively [21,22].

Table 3: IAP recommendations for supplementation of Vitamin D.

Table 4: AAP recommendations for prevention and treatment of Vitamin D deficiency.
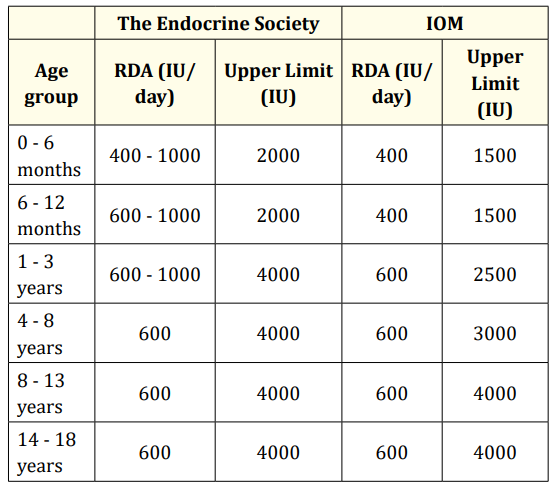
Table 5: The Endocrine Society and the IOM (Institute of Medicine) recommendations.
“Stoss therapy”, derived from the German word stossen, means “to push” [23]. This therapy has been utilised for management of vitamin D deficiency in scenarios where compliance is a concern. It is administered in the dose range of 100,000 - 600,000 units in children over 1 month of age, via oral or intramuscular route. Various studies, with different treatment regimens, have found stoss therapy as an effective way to normalise 25OHD status. Tannous., et al. conducted a study in 151 children and found that no adverse effects related to mega dosing occurred in the stoss therapy group vs the standard treatment group [24]. In a study by Shah., et al. comprising of 42 children with vitamin D deficiency (25OHD < 50 nmol/L), it was found that a total single dose of 150 000 IU significantly increased 25OHD levels, compared to 84000 IU given as 2000 IU/day for 6 weeks (125 and 60 nmol/L, respectively) [7]. Emel., et al. in their study, found that the levels of PTH and ALP were similar in both low-dose vitamin D (2000 IU/day for 6 weeks) and stoss therapies (150,000 IU at once) [25]. Shepherd., et al. discovered a significant rise in mean 25OHD levels amongst children with inflammatory bowel disease who were administered single dose of Vitamin D, ranging from 200,000 to 800,000 IU [26]. The studies have been summarised in table 6.
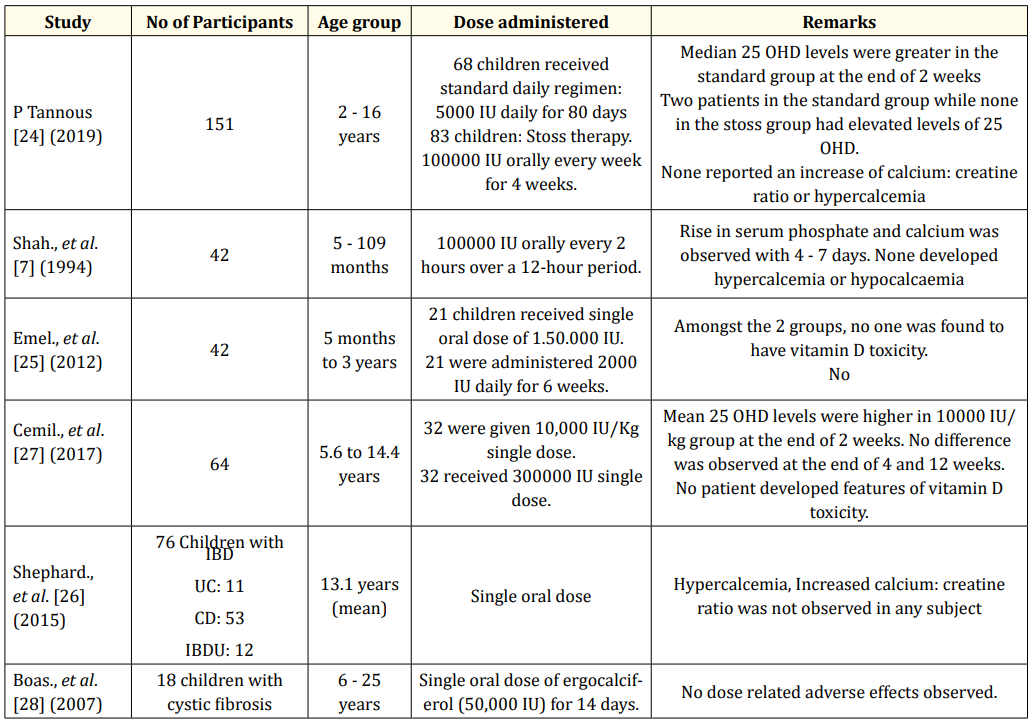
Table 6: Various studies on stoss therapy verses standard regimen.
Vitamin D deficiency has emerged as an unrecognised pandemic. It is an extremely common condition in pediatric population, especially in the malnourished, in those with a chronic illness, limited sun exposure and on chronic drug therapy. Adequate levels are imperative to proper bone maturation during accelerated periods of growth in children. Therefore, knowledge of optimum vitamin D supplementation becomes an essential requisite for all pediatricians. The levels of vitamin D that corroborate with deficiency leading to manifestations are controversial. Levels more > 20 ng/ml are considered sufficient by most societies. Mass screening of vitamin D deficiency is not recommended.
The presence of numerous guidelines is bound to confusion about supplementation and treatment of vitamin D deficiency for various age groups. Daily supplementation is being recommended by most international organisations as well as the Indian Academy of Pediatrics. Stoss therapy was initially used intermittently as prophylaxis (every 3 to 5 months) led to hypercalcemia in 34% of infants. Thus, many pediatricians refused to use this method of treatment. However, many studies, which have compared the standard therapy with the stoss regimen have not observed any adverse effects after administration of mega doses. Stoss therapy may therefore be used to supplement Vitamin D in cases of doubtful compliance and where optimal level of vitamin D have not be achieved despite supplementation.
Copyright: © 2021 Shagun Singh and Roosy Aulakh. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.