I Lemssahli1*, K Hajjout1, M Benajiba2 and A Belmekki3
1Rabat Regional Blood Transfusion Center, Morocco
2National Center for Blood Transfusion and Hematology, Morocco
3Blood Transfusion Service at the Military Hospital, Morocco
*Corresponding Author: I Lemssahli, Rabat Regional Blood Transfusion Center, Morocco.
Received: December 18, 2020; Published: February 22, 2021
Citation: I Lemssahli., et al. “Haemovigilance Recipients at the Rabat Regional Blood Transfusion Center 2015-2019”. Acta Scientific Paediatrics 4.3 (2021): 56-62.
Introduction: Haemovigilance is an element of transfusion safety. Its operating indicators are based on the traceability of transfused blood and the reporting of transfusion incidents.
The objective of this study is to analyze the haemovigilance records returned to the Rabat Regional Blood transfusion center and all RAR notified over a 5-year period, in order to assess the rate of traceability and the incidence of adverse effects of transfusion therapy in healthcare services.
Material and Methods: The number of labile blood products (LBPs) delivered to health facilities is estimated at 432,868 LBPs. During that period, 68,106 LBPs were the subject of feedback and 181 transfusion incidents were reported.
Results: Feedback from delivered LBPs is very low; the traceability rate for LBPs is 20%. Transfusion incidents declaration average rate is 0.41/1000LBPs.
Non-hemolytic febrile reactions and allergic reactions accounted 76% of the reported RARs. Grade 1 accounted 85.34% of RARs. The "serious" reactions (severity grade> 1) represent 10%. These reactions include one case of volume overload, 03 cases of pulmonary edema of which one was fatal, 06 cases of ABO incompatibility of which two were fatal, 03 cases of convulsions, and 05 states of shock of which 02cases required intensive care.
The RARs were secondary to the transfusion of red blood cell concentrates in 70%.
Conclusion: The average rate of the LBPs traceability is 20%. The median cumulative incidence of recipient’s adverse reactions (RARs) is 0.41/1000 LBPs delivered. Febrile non-hemolytic reactions and allergic reactions accounted 76%. Grade 1 totaled 89.4% of RARs. The Serious" reactions (severity grade > 1) represent 10%, which include 1 case of volume overload, 03 cases of pulmonary oedema of which one was fatal, 06 cases of ABO incompatibility, two of which were fatal, 03 cases of convulsions, 05 states of shock, 02 of which required intensive care.
The RARs were secondary to transfusion of red blood cells in 70% cases.
Keywords: Haemovigilance; Feedback; Recipient Adverse Reactions; Labile Blood Products; Blood Transfusion Safety; Legal Obligation
Haemovigilance, term resulting from the fusion of the Greek word “haema” means blood and the Latin word “vigil” means vigilant. The concept of haemovigilance appeared for the first time in France in 1990, almost with the same ideas and the same vision of pharmacovigilance [1].
Haemovigilance is the set of control procedures organized from the collection of blood and its components to the control of recipients, in order to collect and evaluate information on unexpected or adverse effects resulting from the therapeutic use of labile blood products and to prevent their occurrence [2].
Nowadays, haemovigilance systems have been applied in most developed countries, to monitor adverse events and episodes related to donated blood and transfusions. It is recognize as an integral part of the quality management system of a blood transfusion program [3].
In Morocco, haemovigilance started in 1993 at the Ibn Rochd University Hospital in Casablanca; the notification of transfusion incidents was made mandatory by law in 2005 [4].
The development of our haemovigilance system can only be designed if the traceability of labile blood products from donor to actual recipient is obtained [5].
This operation requires close collaboration between blood transfusion center (BTC) and healthcare establishments (HE).
The functioning of haemovigilance is measured through the traceability of LBPs delivered to healthcare establishments (HE) and the declaration of recipient adverse reactions (RARs). This operation requires close collaboration between blood transfusion center (BTC) and healthcare establishments (HE) for the exchange of information between the two establishments.
Haemovigilance can only be conceives if the prerequisite for the traceability of labile blood products, from the donor to the actual recipient, is obtained [5].
The objective of our work is to assess the traceability of labile blood products (LBPs) distributed to the various (HE), to identify and analyze the adverse reactions of recipients declared from January 01, 2015 to December 31, 2019 and compare them with those found in other countries with more experience in this field.
Every year, the Rabat Blood Transfusion Center (BTC) delivers more than 80,000 LBPs to ensure transfusion therapy in more than 60 healthcare establishments (HE), distributed among university hospital center (UHC), public hospitals, private clinics, blood banks.
The database for our study comes from delivery notes (DN) for each labile blood product (LBP) and transfusion incident sheets.
The information collected includes the delivery note number, the product bar code (BC), the donation BC, the patient identity (surname, first name), and the confirmation of the LBPs use.
The data of the transfusion event: clinical manifestations, date and time of the event, the BC of LBPs, quantity transfused, healthcare establishment and the signature of the physician in charge of the transfusion.
The HE is required to return us the transfusion incident sheets, the bag of implicated LBPs and a sample of the patient's blood for the immuno-hematological and bacteriological tests.
From January 01, 2015 to December 31, 2019, the Rabat BTC delivered 432,868 PSL to different care establishments, 61% are RBCs, 22% are Platelets and 17% are FFPs.
Out of 432,868 LBP delivered, the HE have returned the information about 84,722 LBP.
The Histogram1 shows the annual number of LBPs attributed to healthcare establishments and the feedback.
Over the five years, 84,722 LBPs of the total 432,868 LBPs delivered have been traced.
The comparative analysis of the traceability of the number of labile blood products according to years shows a reduction in traceability in 2016 followed by a maximum traceability in 2017, then a decrease in 2018 to stabilize until 2019 (Table 1).
The average traceability rate calculated is 20%. (Table 1) of the public HE 12% and 8% of the private HE, while untraced LBPs represent the vast majority 80%.
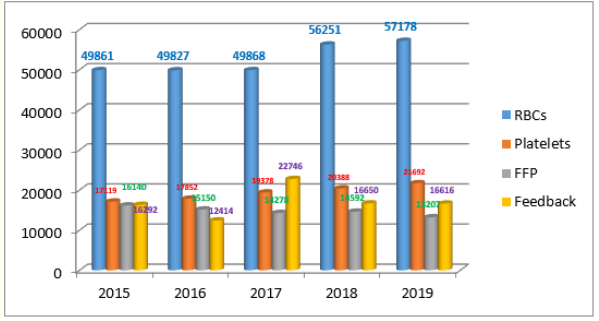
Histogram 1: Delivery of LBPs 2015-2019.
As for recipient adverse reactions (RARs), 181were declared. Analysis of the number declared annually shows great instability (Curve 1).
The patient's average age was 44.3 years with extremes (from 03 Months to 65 Years), 56.7% are females; 60% of reports come from pediatrics haemato-oncology services
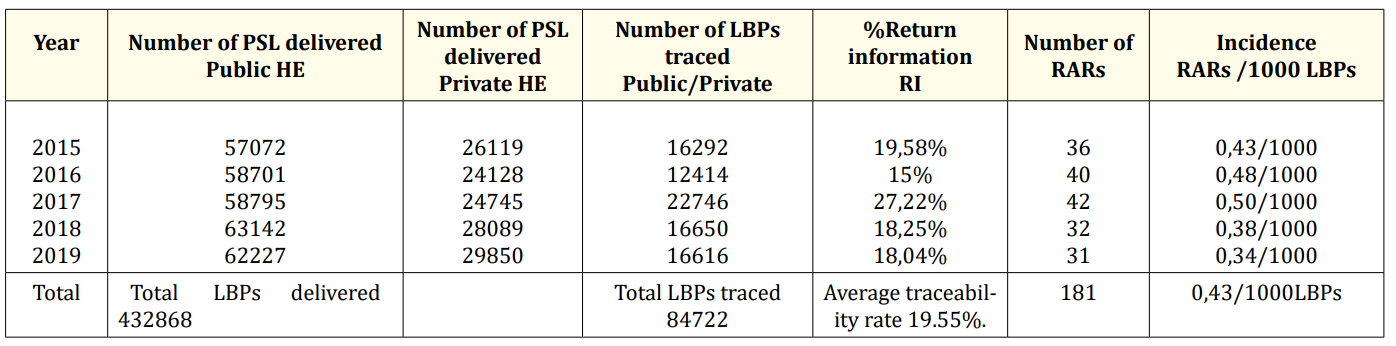
Table 1: Feedback of LBPs delivery 2015-2019
The main reactions reported were febrile non-hemolytic reactions (40.5%), allergic reactions (35.5%), isolated thoracic manifestations (dyspnoea, chest pain, etc.) accounted for (8.15%) and isolated digestive manifestations (abdominal pain, nausea, vomiting (9.5%) (Histogram 2).
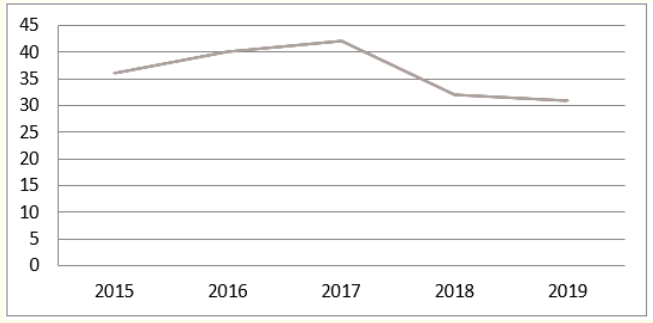
Curve 1: Evolution of the declaration of RAR.

Histogram 2: RARs Distribution.
All reactions were immediate events. The severity grade G1 was approximately 85.34%, the severity grades G2 and G3 were respectively 7.45% and 4.96%; (Histogram 3)
Manifested by ABO incompatibilities (3.31%), states of shock (2.76%), convulsions (1.65%), pulmonary oedema (1.10%), transfusion-related volume overload (0.55%) and possible TRALI (0.55%),
For grade 4 of severity, we report three (03) deaths post-transfusion (1.65%) in 2017 (Table 2).
The Haemovigilance survey shows that red blood cells are clearly more involved in these RARs (70%), followed by platelets in 27% and in third place FFP 3%.

Table 2: Grade 4 Gravity of RARs.
Haemovigilance is an information system on transfusion and part of the transfusion security system [5]. It may only be conceived if the pre-requisite for the traceability of labile blood products from donor to the recipient is obtained [6]. Effective haemovigilance requires close collaboration between the different actors of BTC and HE.
This collaboration remains very unsatisfactory, reflected by the average traceability rate recorded in our study, which does not exceed 20%.
Analysis of the evolution of traceability rates in our series shows low and irregular rates similar to those reported by S. Oudghiri et al in 2010 and 2012 [7].
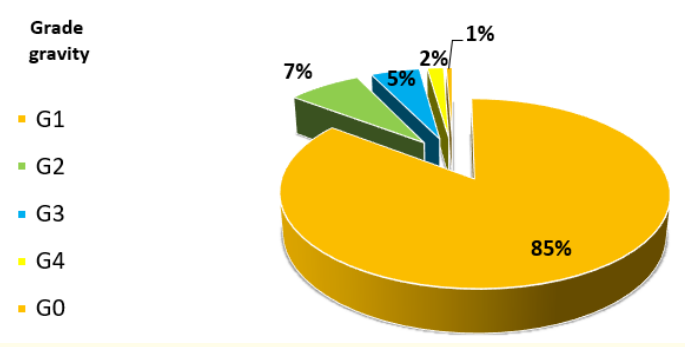
Histogram 3: Severity grade of RARs.
Compared to international data, the traceability is not very significant. The French haemovigilance system reports a traceability rate of 98.9% [9]; in other European countries and in North America, the traceability rate reaches 100% [5, 10].
S.oudghiri et al. have noted that the low rates of traceability are due to the uninvolved health care staff who consider traceability as a workload, an administrative constraint more than a tool to improve the transfusions quality [7].
Most of these dysfunctions are due essentially to the healthcare staff's failure to comply with the legislative requirements on haemovigilance [4].
During the study period, 181 RARs were reported. A cumulative annual incidence shows that the rate does not exceed 0.41 / 1000 LBPs.
Compared to international data, these are very low values [8, 9]. This can only be explained by under-reporting and not by a real decrease in transfusion reactions, probably due to underestimation of minor accidents.
In this study, the main cause of transfusion reactions was febrile non-hemolytic reactions (FNHTR) (40.5%). It is secondary to allo-immunisation against leukocyte antigens [13]. This pathology should remain an exclusion diagnosis and should not be confused with the initial phase of Septic Shock or ABO incompatibility [12].
The FNHTR were all immediate reactions, clinically manifested by hyperthermia and shiver syndrome during transfusion. All patients in the study series had a sudden thermal increase of 1°C often combined with minor subjective symptoms such as feeling cold, discomfort, difficulty with respiration, headache, nausea and vomiting, tachycardia or hypotension, resulting in discontinuation of the transfusion in the majority of LBPs recipients. All of these signs disappeared spontaneously or with symptomatic treatment a few hours after the transfusion was stopped.
The French haemovigilance system evaluates their annual incidence at one case for every 1,632 LBPs transfused and affirms that the leukodepletion has considerably reduced the number of these cases [12.13].
Transfusion of platelets with a short lifetime and deplasmatisation are the most appropriate solutions for preventing iterative FNHTRs [12, 13].
In Morocco, leukodepletion of red blood cells is not systematically applied and only at the clinician's request.
Awareness of the importance of LBPs leukodepletion has contributed significantly to the decrease in NHFTRs compared to the rates published in 2016 [8].
The National Centre for Blood Transfusion and Hematology (NCBTH) policy provides in its 2020-2025 action plan the systematic leukodepletion of red blood cell and the development of germ inactivation [15].
The second highest frequency concerns allergic reactions (35.5%). They appear during or after the transfusion. The pathophysiology of allergic transfusion reactions remains insufficiently understood, often including several factors that may be causally related from the transfusion recipient, the blood donor and the LBPs [16].
The risk of transfusion allergy is highest in the 1-19 age group [17].The Platelets are the main cause of this complication.
The incidence is estimated at one event per 200 platelets transfused [17, 12]. Clinical manifestations of varying severity are reported [12], but most cases are grade 1 [18].
The potential role of plasma in the induction of these events has been reported in American and British studies [5].
The frequency of allergic reactions can be reduced by deplasmatisation of red blood cells, the introduction of a platelet storage solution, leukodepletion and reduction of LBPs storage time [12, 14].
In this series, all sixty-three (63) cases of allergic reactions reported were grade 1 immediate reactions; (41.2%) occurred during platelets transfusion; (31.7%) were due to RBCs transfusion and (26.90%) were caused by FFP transfusion.
The Pediatric haematology-oncology departments reported more than 60% of these reactions. Our results are consistent with the literature.
Of all 181 RARs notified, 10% were "serious" (degree of severity) > 1. Particular importance is attached to two types of incident.
The first incident is poorly known: TRALI, the second is mismanaged: ABO incompatibility.
TRALI: acronym for “transfusion related acute lung injury”, was defined in 2004 by the Toronto consensus conference [12,19] as lesional pulmonary edema occurring within 6 hours after the end of a transfusion and progressing to acute respiratory distress syndrome (ARDS) without any other factor of ARDS ̈ [12,19]. RX pulmonary will reveal bilateral infiltrations corresponding to pulmonary edema [19-22]. To date, the diagnosis of TRALI remains as defined in the Canadian consensus criteria and the : possible TRALI (p-TRALI) is still used in cases where a patient develops mild ARDS associated with a transfusion [20,21].
TRALI has an estimated incidence of almost 1/5,000 labile blood products [20]. It is now the leading cause of post-transfusion death in the United States [20].
The diagnosis of TRALI is based only on its clinical presentation and depends on a high level of vigilance at the patient's bedside, because it is an entity often under-reported [22].
In this study, we report an incidence of 0.007 / 1000 LBPs transfused for three cases of post-transfusion pulmonary edema.
The diagnosis of possible TRALI (p-TRALI) is retained in 02 patients aged 35-58 years respectively, occurred following the transfusion of FFP, the patients presented clinical signs such as fever, dyspnea, tachypnea, pulmonary crackles and hypotension with no radiological signs. The progression was favorable in a few hours with simple oxygen therapy.
The diagnosis of TRALI was reported in a 55-year-old patient who presented radiological signs such as bilateral pulmonary infiltrations. This incident was the result of both FFP and platelets transfusion. The evolution was unfavorable and a death was declared in the hour following the transfusion. The incidence described in our study is much lower than in the French haemovigilance report [18]; it can only be explained by the clinicians' under-reporting.
The second serious incident is ABO incompatibilities that require particular attention, as they are entirely preventable by compliance with good transfusion practices and procedures; they are potentially a source of significant morbidity and mortality. They result from an incorrect administration of LBPs, which is the consequence of a mistake or a series of mistakes along the transfusion chain: error of attribution, incorrect identification of the patient/ LBPs.
Data in the literature show that in over two thirds of cases, the main source of dysfunction is the failure of the hospital to provide pre-transfusion control at the patient’s bedside, which is the last safety lock [23].
Over the study period, six cases of ABO incompatibility were declared, with an incidence of 0.014/1,000 LBPs (05 were children between 1 and 12 years and 01 were female 79 years old). 2.20% are in grade 2 and 1.10% in grade 4 (Table 2).
In our series, the incidence of ABO incompatibility is higher than that reported by the Casablanca and Rabat team [5,8] and is similar to the incidence reported in the Maghreb [24,25].
Our Haemovigilance investigation of the 06 cases of ABO incompatibility found that the incompatible transfusions resulted both from human error and failure to comply with good transfusion practices as described in the literature [12,23].
Awareness raising and continuous training of medical and paramedical staff is a key element of transfusion safety.
Providing a good feedback and a better notification of RARs is a challenge for the haemovigilance system.
Haemovigilance is a Quality process to improve and increase transfusion safety. It covers and monitors all activities of the transfusion system.
A considerable delay in developing the national haemovigilance system as expressed by the low involvement of healthcare establishments in the haemovigilance process manifested by a very low feedback rate and under-reporting of transfusion incidents;
It is imperative to:
Establish a development strategy to deal with the difficulty of implementing a haemovigilance system. Review the national organization of the haemovigilance system.
Implement corrective actions oriented towards training and regular awareness raising of healthcare staff.
Develop and dynamite communication between the different stakeholders.
Develop the support of the RARs declaration, and set up a software via a network between the Blood Transfusion Centre and the Healthcare Establishment.
Secure data gathering and facilitating the work of the reporters and haemovigilance correspondents.
Copyright: © 2021 I Lemssahli., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.