Poorva Gohiya*
Assistant Professor, Department of Paediatrics, Gandhi Medical College, Bhopal, Madhya Pradesh, India
*Corresponding Author: Poorva Gohiya, Assistant Professor, Department of Paediatrics, Gandhi Medical College, Bhopal, Madhya Pradesh, India.
Received: November 16, 2020; Published: February 11, 2021
Citation: Poorva Gohiya. “Neurodevelopmental Outcome of Preterm (32 - 36 weeks) Neonates Based on Developmental Assessment Scale for Indian Infants”. Acta Scientific Paediatrics 4.3 (2021): 35-41.
Objectives: To assess the neurodevelopment of preterm (32 - 36 weeks) NICU graduates till 1 year of age and factors affecting it.
Methods: This is a prospective cohort study where 160 preterm babies of gestational age 32-36 weeks were enrolled using purposive sampling for study and were followed up for a period of one year.
All the details of hospitalisation were recorded on a pretested proforma. These babies were followed till 1 year of age. Their development was assessed with Developmental Scale for Indian Infants (DASII).
Results: The mean gestational age was 33.92 ± 1.378 weeks. Study population comprises 57.5% females. The mean motor development quotient (DQ) was 73.3 ± 9.2 and motor delay observed was 35.2%. The mean mental DQ was 80.5 ± 8.2 and the mental delay was 13.2%.
Conclusion: We observed a neurodevelopmental delay of 34% in the preterm babies after gestational age correction. Using DASII scale, a significant delay was noted in memory, social interaction and language domains. Assessment of neurodevelopment and serial monitoring would guide to start early intervention to minimise the delay at an earlier stage.
Keywords: DASII; WHO;
India ranks first in the list of preterm births fact sheet released by WHO in 2016. WHO defines preterm babies as those born alive before 37 completed weeks of gestation [1]. The incidence of preterm delivery and the survival rate of preterm babies is rising due to new medical technologies. Late preterm infants represent a sizable population of preterm infants. Approximately 70-75% of preterm infants are born between 34 - 36 weeks of gestation which account for all births [2].
In preterm babies, prenatal, perinatal and postnatal determinants can give rise to adverse neurological outcomes through complex and causal pathways via hypoxic or ischemic inflammation. Early birth has an influence on brain development and timing of neurobiological process. These processes include neuronal migration, axon and dendrite sprouting, synapse formation, persistence of transient structures involved in anatomical segregation of thalamic axons from lateral geniculate nucleus in visual cortex [3].
There has been a shift in the distribution of births away from term and post-term towards preterm. Among this births more than 75% are late preterm babies. Late preterm babies account for one third of all NICU admissions. Although the mortality rate has decreased due to improved neonatal care the statistics of neonatal morbidity and poor neurodevelopmental outcomes has increased [2].
Apart from their fight for survival, these preterm babies suffer from various postnatal complications including environmental and metabolic complications which further are responsible for the developmental delay. In this study attempt has been made to assess the neurodevelopment in preterm babies using Developmental Scale for Indian Infants (DASII) and the risk factors contributing to it.
Prospective observational study.
March 2017 to June 2018.
NICU, Department of Paediatrics, Tertiary Care Teaching hospital.
All the preterm babies belonging to 32 to 36 weeks of gestation admitted in NICU irrespective of their indication of admission fulfilling the inclusion criteria
A total of 160 preterm babies of gestational age 32-36 weeks discharged from March 2017 to June 2017 were enrolled for study and were followed up for a period of one year. The sample size was calculated using the following formula.
N= 4PQ / L2
taking P (prevalence) as 10% (1)
L = allowable error which was taken as 5%
Q = 1 – P
A calculated sample is 138 + additional 10% for follow-up study. So a sample calculated was 160. Sample collection was done by purposive sampling.
After obtaining ethical clearance from Institute’s ethical committee, an informed written consent from the parents of all neonates fulfilling the inclusion criteria was taken in a local language. A total of 160 preterm neonates admitted fulfilling the inclusion criteria were selected using purposive sampling and were followed up till 1 year of age (Figure a).

Figure a: Study flow chart.
History from the mother regarding antenatal visits, last menstrual period, perinatal period, maternal diseases, was taken and recorded in the pretested proforma. The details of indication for admission, anthropometry, condition on admission were recorded. Gestational age was assessed using New Ballard score [4]. Treatment given during hospitalization, course of stay and special procedures done were recorded.
Detailed feeding history and the day at which rooming in was done were also noted. Baby’s weight, length and head circumference were again noted at the time of discharge. Advice regarding feeding, warmth, prevention of infection were given during stay and at discharge.
During every follow-up anthropometry of the babies was done by a single observer. The age of the baby was adjusted as per the weeks of prematurity (i.e.) corrected gestational age. The neurodevelopmental screening test used was Trivandrum Development screening chart (TDSC) [5]. Any delay in the neurodevelopment was confirmed by using Developmental Assessment Scale for Indian Infants (DASII) [6]. All babies were screened at baseline and 12 months using TDSC. Any developmental delay observed was confirmed and assessed using DASII which was conducted at admission and at 12 month.
Data was compiled using Ms Excel and analysed using IBM SPSS VERSION 20 software. Quantitative data was expressed as mean whereas qualitative data was expressed as number and percentage. Chi-square test and multiple regression analysis was done. P value less than 0.05 was considered significant.
About 123 (76.9%) neonates were admitted at less than 24 hours of age. The average age at admission was 12.59 ± 22.51 hours (Median age -12.50 hours). The mean gestational age of admission was 33.92+1.378 weeks. Two thirds of the study population were females (57.5%) (Table 1). The mean NICU stay was 6.36 ± 3.6 days. Out of 160 neonates, neonatal death was observed in 1 case whereas 159 neonates were discharged.

Table 1: Demographic distribution.
The mean weight at admission was 1.62 ± 0.49 kg and at 12 months was 2.95 ± 0.64 kilograms. The mean length at admission was 34.8 ± 1.30 centimetres and at 12 months was 45.71 ± 33.52 centimetres. The mean head circumference at admission was 30.1 ± 1.48 centimetres and at 12 months was 32.94 ± 1.25 centimetres. SGA neonates contributed to 12.5% of the study population (n = 20), of which 6.9% neonates showed a delay in their development. Vaginal delivery was the most common mode of delivery with 81.9% (n =134). Sixty percentage of the mothers were of the age group between 20 to 25 years. Fifty five percentage of mothers were anemic with haemoglobin less than 10 g% (Table 1).
The mean actual and corrected motor DQ observed was 73.3 ± 9.2 (Median- 75) and 79.7 ± 8.6 (Median- 82) respectively. In the motor clusters, neck, body and manipulation clusters showed no delay and the infants have attained more than 75 percentile in all 3 clusters. Of the total neonates studied 27% of 159 infants had a delay in locomotion I (n = 43; p= 0.002) which was highly significant (Table 2, Figure 1).
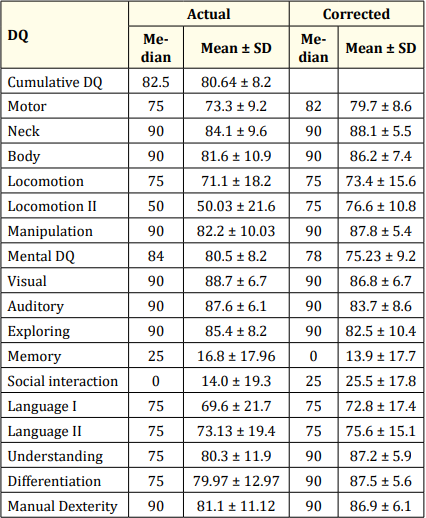
Table 2: Median DQ for actual age and corrected age.
The mean mental DQ actual and corrected in present study were 80.5 ± 8.2 (Median- 84) and 75.23 ± 9.2 (Median 78) respectively On individual domain analysis, in memory 154 infants had a DQ<75th percentile which contributes to 96.8% delay. Social interaction showed a delay of 95.59% with 152 infants having DQ< 75th percentile (p < 0.001) which is highly significant. Language II showed a delay of 18.9% delay with 30 infants DQ<75th percentile and the p value is <0.001 with high significance. Language I also had a delay 32.7% with 52 infants DQ<75th percentile with p value < 0.001 (Table 2, Figure 2).
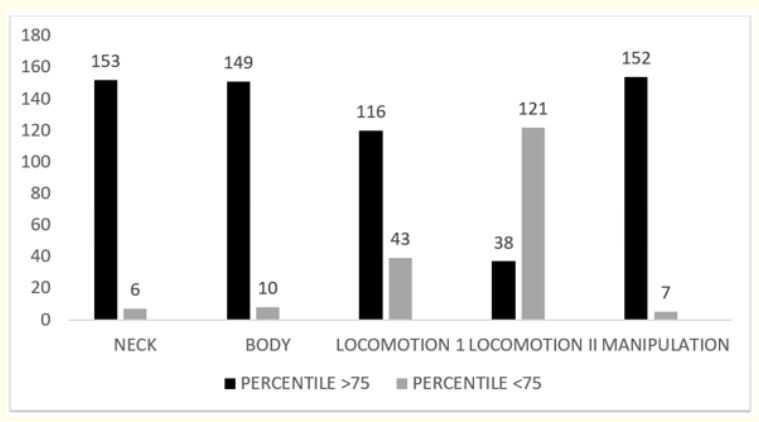
Figure 1: DQ in individual motor clusters.
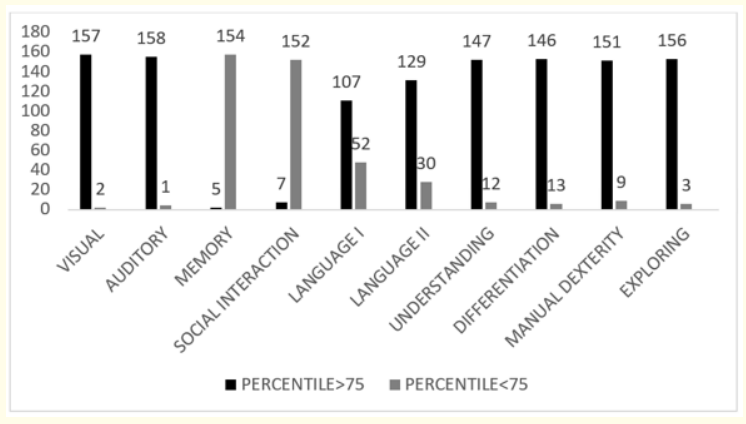
Figure 2: DQ in individual mental clusters.
In present study, DQ was significantly associated with various risk factors (p < 0.001). The present study documented a statistically significant association of gestational age with body component of actual motor DQ (p < 0.05) whereas no such association was observed for any of the components of DQ (p > 0.05). Also we observed no association of gestational age with corrected motor DQ (p > 0.05).
Similarly, no significant association of actual motor DQ was observed with birth weight whereas neck and body component of corrected motor DQ were significantly associated with birth weight (p < 0.05).All the components of mental DQ were not associated with gestational age in present study whereas auditory component of corrected mental DQ was significantly associated with gestational age (p < 0.05). However, mental DQ both actual and corrected were observed to have no statistically significant association with birth weight (p > 0.05).
Out of 160 neonates, death was documented in 1 neonate, however, 159 neonates were followed up till 12 months. Of the 159 neonates (n = 56) had a DQ less than 70 which contributed to 35.2% of delay.
In the motor clusters, neck, body and manipulation clusters showed no delay and the infants have attained more than 75 percentile in all 3 clusters. But locomotion I and II clusters have shown significant delay. About 27.0% of 159 infants had a delay in locomotion I (n = 43; p= 0.002) which was highly significant. And 76.1% of 159 neonates had a delay (< 75th percentile) in locomotion II cluster which is (n = 121, p = 0.001) highly significant. The locomotion II cluster contains 13 items and at 1 year of age none of the item will be passed by a normal child. Thus the locomotion II cluster should not be considered for assessment till 1 year of age.
Kanya., et al. (2013) [7] in their study selected very low birth weight and extremely low birth weight preterm babies where the motor delay was 25%. DASII was used as assessment tool and preschool Behaviour checklist (PBCL) was used till a corrected age of 2 years. This delay percentage was lesser than the motor delay (38.36%) observed in our study. Various neonatal morbidity factors observed in the study and a short follow-up period (1 year) could be the factors contributing for the higher delay percentage in our study. The mean mental development quotient (DQ) observed in this study was higher than the motor DQ and a similar result was observed by Kanya., et al. also [7].
Soleimani., et al. [8] in his review said that preterm neonates are more prone for neuromotor disability like cerebral palsy, hearing impairment and motor impairments like coordination, gait abnormalities. He did not specify about the minor changes in motor impairment. Similarly locomotor disability was observed in present study.
A comparative study of very low birth infants and term normal birth weight infants till 12 months of corrected age was conducted to evaluate their growth and neurodevelopmental outcome by Manoj., et al. and he had done assessment using DASII. Anthropometric measurements were recorded and z scores were computed at birth, discharge, 1, 3, 6, and 12 months. VLBW infants had significantly lower Z-scores for weight, length and HC at one year corrected age as compared to NBW infants (P = 0.01, 0.04 and 0.001, respectively). DQ at 12 months was significantly lower in VLBW infants (91.5 ± 7.8) than NBW infants (97.5 ± 5.3) (P < 0.001). He concluded that LBW infants though catch up physical growth later in infancy, they continue to lag in their neurodevelopment at 1 year of corrected age and suggests a multipronged approach to improve growth and development of these infants [9].
The mean mental DQ observed was 75.23 ± 9.2 at corrected gestation age. While the chronological age DQ was 80.5 ± 8.2. Fifty six of the total 159 neonates showed a delay (<70 DQ) which results in 35.2%. Six of the 10 mental clusters showed normal development in maximum neonates which includes visual, auditory, understanding relation, differentiation, exploring and manual dexterity. The clusters which showed delay were memory, social interaction, language I and language II.
In memory 154 infants had a DQ <75th percentile which contributes to 96.8% delay. Social interaction showed a delay of 95.59% with 152 infants having DQ < 75th percentile (p < 0.001) which is highly significant. Language II showed a delay of 18.9% delay with 30 infants DQ<75th percentile and the p value is <0.001 with high significance. Language I also had a delay 32.7% with 52 infants DQ <75th percentile with p value < 0.001.
The present study documented a statistically significant association of gestational age with body component of actual motor DQ (p < 0.05) whereas neck and body component of corrected motor DQ were significantly associated with birth weight (p < 0.05).
Louthrenoo., et al. [10] stated that the neurodevelopment at 12 months of age of preterm were not the same as that of term infants in his study where he used Bayley scales Of Infant development (BSID - II). He also stated that identification of delay at 1 year is an early indicator to start intervention. In this study delay at an earlier age (10 months) in individual clusters of mental and motor development was identified. It indicates better sensitivity of the tool used in the study and DASII can help to improve neurodevelopmental outcome as domain specific interventions can be started early.
Incidence of prematurity was significantly higher in children with speech delay (p < 0.05) in a retrospective study done by Nandita., et al. [11]. Pierrat., et al. did a population based cohort study and assessed preterm babies till 2 years of age in 3 gestational groups. Mental scoring done with ASQ score showed an average of 5.6% delay with individual domain score significantly low in communication and personal social interaction score. Similar results have been observed in our study. Pierrat., et al. concluded that the rate of cerebral palsy though decreased, the risk of developmental delay was high, even in moderately preterm children [12].
Kanya., et al. [7] reported that the behavioural outcome in their study showed that large number of VLBW babies had an abnormal high score and all ELBW babies had high PBCL score. These babies need long term behavioural follow up to pick up attention deficit hyperactivity disorders at the earliest. Our study also described deficit in social interaction in preterm infants which can lead further to various childhood behavioural disorders. Due to short time period of the study and small study population such correlation could not be observed in present study.
Zhang., et al., Shah., et al. and Domenico., et al. also conducted similar follow up studies and concluded saying that preterm infants discharged from NICU are at a high risk group of neurodevelopmental disablement. Early intervention can improve the neurodevelopmental outcome of preterm infants at high risk [13-15]. Carlo., et al. published a review article regarding neurodevelopmental outcome of preterm babies where he reviewed 10 years neurodevelopment articles. This review stated that preterm babies with diffuse white matter degeneration show about a 33% reduction in cortical and deep gray matter volume reduction in complexity of cortical folding. The most involved areas are basal ganglia, amygdala, thalamus, hippocampus and brainstem. Neurologic abnormalities, learning difficulties, poor scholastic achievement have been found [16].
Most common neonatal morbidity factor (Table 2) observed in the study population was RDS (respiratory distress syndrome) affecting 78 babies (48.75%) followed by sepsis (15.6%), neonatal hyperbilirubinemia and IUGR (intrauterine growth retardation) affecting 18 (11.3%) and 16 (10%) neonates respectively.
Following up, 11 of the 78 neonates with RDS showed a delay <70 contributing to 14.1% to delayed cohort, while early onset sepsis contributed to 16% delay which is depicted in table. Sepsis as a risk factor at birth, was found to independently determine educational status of the child in the Pune LBW study done by Sudha., et al. [17,18]. Our result also observed a direct relation of sepsis with developmental delay. A study in a cohort of 110 infants conducted by Katherine., et al. confirmed neonatal infection heightens VPT infants’ risk for neurodevelopmental impairment. White matter abnormalities appears to be an important intervening factor linking infection and severe motor and IQ impairments [19]. Neonatal sepsis was an independent risk factor for neuromotor development impairment in premature infants in the age range studied by Ferreira., et al. [20], but there was no association with mental development. His results suggested that children who develop either confirmed or clinical sepsis in the neonatal period have a differentiated follow-up of their neuromotor development.
Preterm neonates of our study population showed a developmental delay of 34%. The individual milestones like memory, language, social interaction and locomotion are affected in more than 50% of neonates taken into study. Delay in cognitive and locomotor skills show subtle changes at earlier age and so these milestones require close observation and serial assessment of infants.
Our study had shown that delay in individual domains of motor and mental development can be diagnosed at an early age by using DASII and thus domain specific early intervention therapy can be planned. This will help in decreasing the overall neurodevelopmental delays and thus morbidity and overall outlook of preterm babies.
Copyright: © 2021 Poorva Gohiya. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.