Foli Agbeko1,2*, Mawouto Fiawoo1,3, Enyonam Tsolenyanu1,3, Kokouvi Evenyo Abalo3, Magnoulelen N’zonou4, Sollim Talboussouma5, Homba Daké Batalia3, Rollin Arnaud Djomaleu3, Rachel Bayahou Kérékou2, Manani Hemou2, Mazama Pakoudjare2, Koffitsè Essèboè Sewu6, Bayaki Saka7, Edem Koffi Djadou1,3, Kokou Nadiedjoa Douti1,2 and Yawo Dzayissé Atakouma1,3
1Département de Pédiatrie/Faculté des Sciences de la Santé/Université de Lomé, Togo
2Service de Pédiatrie, CHU - Campus, Lomé, Togo
3Service de Pédiatrie/CHU Sylvanus Olympio, Lomé, Togo
4Service de Pédiatrie/Hôpital de Bè, Lomé, Togo
5Service de Pédiatrie/CHU Kara, Kara, Togo
6Centre Africain de Recherche en Epidémiologie et en Santé Publique de Lomé, Togo
7Service de Dermatologie, CHU Sylvanus Olympio, Université de Lomé, Togo
*Corresponding Author: Foli Agbéko, Département de Pédiatrie/Faculté des Sciences de la Santé/Université de Lomé, Togo.
Received: December 21, 2020; Published: February 11, 2021
Citation: Foli Agbeko., et al. “Impact of Human Rotavirus Vaccine on Acute Gastroenteritis Among Children of 0-59 Months in Sokodé (Togo) ”. Acta Scientific Paediatrics 4.3 (2021): 17-23.
Background: Acute Gastroenteritis (AGE) was one of the leading causes of child morbidity and mortality worldwide. Rotavirus is the leading cause of diarrhoea in children under five years, leading to severe dehydration. The monovalent rotavirus vaccine Rotarix® was introduced into the Expanded Programme on Immunization (EPI) in Togo in 2014. The aim of the study is to measure the impact of Rotavirus vaccination on Acute Gastroenteritis (AGE) in children under 5 years of age at the Regional Hospital of Sokodé in Togo.
Methods: This was a quasi-experimental "before and after" study. It compared the morbi-mortality linked to AGE in children under 5 years of age, for the period before the introduction of the rotavirus vaccine (Period 1 or P1: year 2013 and 1st half of 2014) and the period after (Period 2 or P2: 2nd half of 2014 and year 2015) in the paediatric ward of the Regional Hospital of Sokodé, Central Region, Togo. Rotavirus was detected by enzyme-linked immunosorbent assay in stool samples that were sent to Lomé as part of a sentinel surveillance.
Results: From 2013 to 2015, 365 records of children under 5 years of age were included (nP1 = 289; nP2 = 76). The average age of the children was 20.79 ± 10.08 months in P1 and 20.77 ± 9.46 months in P2, without significant difference (p = 0.9884). For both study periods, children under 24 months of age accounted for 3/4 of the AGE cases. The M/F sex ratio was 1.16 in P1 and 1.38 in P2 (p = 0.5068). The prevalence of AGE registered in hospital was significantly reduced from 4.80% to 1.20% (p = 0.000) after vaccine introduction. The mortality rate related to AGEs decreased from 0.12% to 0.05% with no significant difference (p = 0.1998). Rotavirus was responsible for 72.30% of the AGE in Sokodé.
Conclusion: The rotavirus vaccine had a significant positive impact on the morbi-mortality of AGE in children in Sokodé. The education of the population must reinforce adherence to the EPI and good food and hygiene practices.
Keywords: Gastroenteritis, Rotavirus, Vaccine, impact, Togo
AGE: Acute Gastroenteritis; CHU: Centre Hospitalier Universitaire (University Teaching Hospital); IPE: Expanded Programme for Immunization; WHO: World Health Organization.
Acute Gastroenteritis (AGE) was one of the leading causes of child morbidity and mortality worldwide [1]. Globally in 2013, 215,000 deaths of children under 5 years of age occurred as a result of rotavirus diarrhoea, nearly half of which occurred in Sub-Saharan Africa [1]. Rotavirus is the leading cause of diarrhoea in children under five worldwide, leading to severe dehydration [1]. The high morbidity and mortality associated with rotavirus-associated AGE has made vaccine development one of the global public health priorities for child protection [2]. Since 2006, two rotavirus vaccines (RotaTeq® and Rotarix®) have been licensed and recommended for use in immunization programmes in Europe and the Americas, and were extended to all countries by WHO in 2009 [3]. Between 2012 and 2016, approximately 43.8 million children in Africa were vaccinated against rotavirus [4].
In Togo, the prevalence of acute diarrhoea in children under 5 years of age was 15% in 2014 [5]. Diarrhoea was responsible for about 10% of deaths in children under five [6]. Rotavirus killed more than 1000 children under five years of age in Togo each year [7]. About 54% of all hospitalizations due to diarrhoea in children under 5 years of age are caused by rotavirus [8]. The majority of all rotavirus diarrhoea (92%) occurred in children under 2 years of age [8]. The monovalent rotavirus vaccine Rotarix® was introduced into the Expanded Programme on Immunization (EPI) in Togo in 2014 [9]. Rotavirus vaccine coverage was 92% in 2018 [9]. Two studies on the positive impact of rotavirus vaccination on AGE were conducted by Tsolenyanu., et al. [10,11]. All of them concerned the capital city of Lomé and its surroundings. To date, in our knowledge, no studies have been carried out on this subject outside this Maritime region, in the northern parts of Togo. It is in this sense that the present work, which aims to measure the impact of rotavirus vaccination in Sokodé in the Central Region of Togo, is part of this work. It will make it possible to analyze the evolution of the morbi-mortality of AGE in children under 5 years of age hospitalized at the Regional Hospital of Sokodé from 2013 to 2015.
This was a quasi-experimental «before and after» study conducted in the sanitory district of Tchaoudjo, Central Region (Togo). The population of the Central Region was 742,661 inhabitants in 2018. It compared the morbidity and mortality related to AGE in children under 5 years of age for two 18-month periods: the period before the introduction of the rotavirus vaccine (P1) from January 1, 2013 to June 30, 2014 and the period after (P2) from July 1, 2014 to December 31, 2015. Included in the study were records of children under 5 years of age who were hospitalized for AGE (defined as ≥3 liquid or semiliquid stools per 24 hours lasting <14 days, vomiting, with or without fever) according to the WHO generic protocol on sentinel surveillance for rotavirus gastroenteritis [13]. A history of fever and a temperature above 38°C, an episode of convulsions were also as associated signs. The classification of dehydration is based on criteria formulated by the WHO in children. The presence of blood in the stools and co-morbidity (malaria, undernutrition...) were the criteria for non-inclusion. This was an exhaustive sampling. The parameters studied were sociodemographic (age, sex, period of occurrence and origin), prevalence, mortality rate, clinical data (notion of fever, vomiting, severity of dehydration and seizures) and biological data. This study was part of a one-off partnership with the sentinel surveillance sites for rotavirus diarrhoea, all located in Lomé (Sylvanus Olympio Teaching Hospital, Bè Secondary Hospital). A sample of stools had been collected in 2013 and 2014 (before the introduction of the vaccine). For the collection, the children were placed on sterile jars. Stool samples were collected in sterile containers with screw caps that were properly labelled. The enzyme-linked immunosorbent assay test (IDEIARotavirus, OXOID) was used for the detection of rotavirus in stool samples that were sent to Lomé (sentinel laboratory of the Sylvanus Olympio Teaching Hospital). Trained medical students helped collect the samples. The data were entered into a database designed under Epi data version 3.1 software. The data were entered into a database designed under the Epi data version 3.1 software. The statistical analysis was done with R Studio software version 3.4.3. The various parameters collected were compared between the pre-vaccination period (P1) and the post-vaccination period (P2). The statistical tests used were the Student test for the comparison of 2 quantitative variables and the Chi-2 or Fisher test for the comparison of 2 qualitative variables. A value of p < 0.05 was significant. We received an authorization from the Prefectural Health Directorate, on which the regional Hospital of Sokodé depends, allowing access to medical records. The anonymity of the medical records was respected.
Between January 2013 and December 2015, a total of 12,419 children under the age of 5 years were hospitalized in the paediatric ward of the Regional Hospital of Sokodé. There were 6,044 and 6,375 registered for P1 and P2 respectively. Among the 12,419 hospitalized children’s files, 365 children were retained for AGE (nP1= 289 or 79.20% and nP2=76 or 20.80%).
The prevalence of AGEs decreased significantly by 75%: 4.80% (289/6,044; CI 4.26-5.36) to 1.20% (76/6,375; CI 0.95-1.50) from P1 to P2 (p = 0.000).
The mean age of children with AGE was 20.79 ± 10.08 months in P1 and 20.77 ± 9.46 months in P2, with no significant difference (p = 0.9884). For both study periods, children under 24 months of age accounted for 3/4 of the cases of AGE. Male children were the most affected by AGE in both series (sex ratio = 1.16 in P1 and 1.38 in P2), with no significant difference (p = 0.5068).
The majority of AGE cases were observed during the months of January (39.50%) and February (20.60%). The peak of AGE cases was in January 2013 (64 cases) and January 2014 (47 cases). A zero incidence was recorded between August and October 2015 (Figure 1).
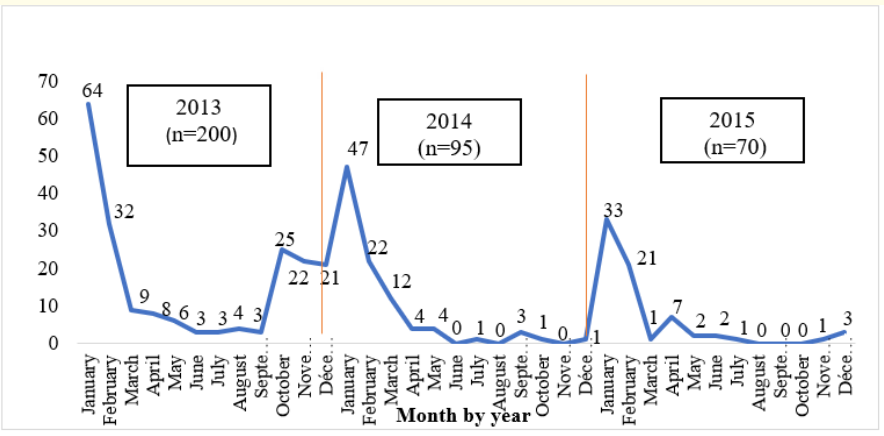
Figure 1: Distribution of AGE cases by month and year.
According to the origin, the majority of children presenting AGE were from the Sokodé agglomeration with respectively 72.30% for P1 and 81.60% for P2, without significant difference (p = 0.1004).
The symptoms most frequently associated with diarrhoea were fever and vomiting with 96.50% and 86.50% respectively in P1 against 65.80% and 85.50% in P2. Approximately one child in ten (9.70%) had convulsed in an AGE setting in P1 (Table 1).
The proportion of AGE cases with severe dehydration varied significantly from 13.80% in P1 and 3.90% in P2, a reduction of 65% (p = 0.0379). No dehydration was observed in 56.6% (P2) vs 46.4% (P1).
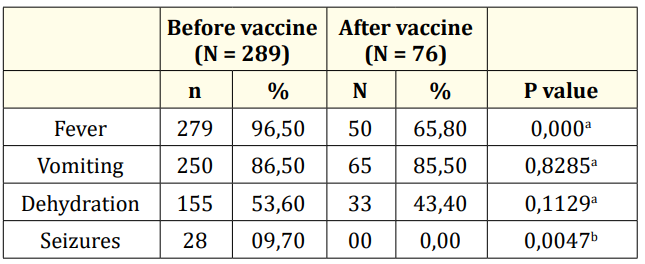
Table 1: Distribution of AGE cases by clinical signs and vaccine introduction period. a= Chi-2 test of independence; b= Fisher’s Exact Test.
Of the 47 stool samples collected for ELISA, 34 were rotavirus positive or 72.30%.
There was a non-significant reduction from 0.12% (7/6,044; CI 0.05-0.25) to 0.05% (3/6,375; CI 0.01-0.15) in the mortality rate of children under 5 years of age hospitalized for AGE (p = 0.1769). The specific mortality rate for children under 5 years of age hospitalized for AGE decreased from 57% (1.26% CI [0.55-2.69] to 0.54% CI [1.02-11.88]) with no statistically significant association (p = 0.1998).
The introduction of the monovalent rotavirus vaccine has demonstrated a significant reduction in hospitalizations of children under 5 years of age suffering from AGE in Sokodé. The prevalence of AGE had decreased by 75% in the 18 months following the introduction of the vaccine (from 4.80 to 1.80%, p = 0.000).
Available studies in Africa and worldwide on the impact of rotavirus vaccines confirm a post-vaccination reduction in hospitalizations related to AGE: 23% in the first year after vaccine introduction and 53% in the second year [11], 65.40% in South Africa [14], 52% in Ghana within two years after vaccine introduction [15], 48.20% in Malawi [16], 44% in Burkina Faso [17], 83% in Belgium [18] and 96% in the United States [19]. The differences observed between the series can be attributed to the methodology used, but all confirm the positive impact of the rotavirus vaccine. The monovalent rotavirus vaccine Rotarix® contains a live attenuated human rotavirus that replicates in the small intestine and produces immunity that protects against the main cause of AGE in children.
The mean age of the children was 20.79 ± 10.08 months in P1 and 20.77 ± 9.46 months in P2, with no significant difference (p = .9884). For both study periods, children under 24 months of age accounted for 3/4 of the AGE cases. These results are superimposable on different studies of rotavirus AGE in which children under the age of 2 years were the main victims (≤ 24 months). Tsolenyanu., et al. [8] in Togo in 2014 reported that 92% of children hospitalized for AGE were less than 2 years old. This is also the case in the study presented by Ezzine [20] in Morocco with 79.40% in 2009 and 76.80% in 2014.
Children were more affected in the months of January and February. This period in Togo corresponds to the dry and harmattan season, a dry, dusty and cold wind from the Sahelian zone blowing towards the southwest and maintaining microorganisms. This is consistent with the seasonality of rotavirus AGE noted in the literature [8,11,17,21-25].
The proportion of AGE cases associated with dehydration decreased from 53.60% to 43.40% in the 18 months following the introduction of the vaccine. Similarly, there was a decrease of about 65% (from 25.80% to 9.10%) in cases of severe dehydration. These results are consistent with those of Ezzine [20] in Morocco in 2014, who reported a 50% reduction in cases of severe dehydration in the two years following vaccine introduction.
Rotavirus accounts for 72.30% of the causes of AGE in stool samples collected. This frequency is similar to that of Mwenda., et al. [26] in 2007 who found 74% rotavirus in a stool sample of 39 children. According to studies conducted by Tsolenyanu., et al. [8,21] in Lomé, Togo, rotavirus was the main cause of diarrhoea with 48.60 in 2014 and 49.10% in 2016. Sangaji., et al. [22] in Congo in 2012 reported a rotavirus prevalence of 53.8%. Ndze., et al. [27] in Cameroon in 2011 estimated a prevalence of 42.8%. In a prospective study conducted in five European countries (France, Germany, Italy, Spain and the United Kingdom) in children under 5 years of age in 2009, rotavirus accounted for 56.2% of all hospitalizations for acute gastroenteritis, ranging from 33.2% in Italy to 64.4% in France [28]. A study conducted in 2014 by Operario., et al. [29] on the etiology of severe acute watery diarrhoea in children in the global quantitative PCR rotavirus surveillance network noted that rotavirus was the most detected (40.3%), norovirus (6.2%), Cryptosporidium (5.8%), Shigella (4.7%), Escherichia coli (4.2%), adenovirus (4.2%). These data show that rotaviruses have an incidence that varies between and within countries, but remain the main etiological agent in AGE of children worldwide.
The mortality rate fell from 0.12% to 0.05%, a non-significant reduction of 58% (p = 0.1769). Richardson., et al. in Mexico, reported a 46% reduction in the mortality rate for acute diarrhoea in children under 5 years of age vaccinated in the years following the introduction of rotavirus vaccine [30]. For Carmo., et al. in Brazil in 2011, the largest reductions in mortality (28%) were observed in children under 2 years of age [31]. In Malawi, a 34% reduction in mortality from childhood diarrhoea followed the introduction of rotavirus vaccine in 2015 according to Bar-Zeev., et al. [32]. A study in Senegal by Diop., et al. in 2015 estimated that rotavirus vaccine would reduce rotavirus deaths by 42% [33].
Within the limits, the Regional Hospital of Sokodé is not a sentinel surveillance site (only existing in Lomé). Not all of the children included had had faeces collected for virological testing, only one sample had been collected and tested. Stool samples could not be collected after the introduction of the vaccine for logistical reasons. Similarly, this study did not identify yet all of the different strains of rotavirus present in Sokodé and the Central region. This is part of our research perspective for the future.
This quasi-experimental «before and after» type study of the introduction of the rotavirus vaccine interested the pediatrics department of the Regional Hospital of Sokodé. It enabled the collection of 365 files of children under 5 years of age hospitalized for AGE from January 2013 to December 2015. The results showed that the prevalence of rotavirus gastroenteritis was high in Sokodé (72.30%) before the introduction of the vaccine. The introduction of the monovalent rotavirus vaccine Rotarix® in June 2014 has had a positive impact on AGE. The hospital prevalence of AGE decreased by 75% and the mortality rate by 58% between 2013 and 2015. The education of the population must reinforce adherence to the EPI and good food and hygiene practices.
We have obtained a study authorization from the chief doctor of the health district of Tchaoudjo and the executive health team of the Central Region in Togo (ref 07/2016/PED/ TCHAOUDJO). This study was also approved by the head of the Department of Pediatrics of the Faculty of Health Sciences of the University of Lomé, Togo. We used the clinical records of hospitalized patients anonymously.
Not applicable.
Extracted data are with the authors and available for sharing on request.
The authors declare that they have no competing interests.
None.
FYA and MF contributed of the development of the protocol, coordinated the field activities, edited the manuscript, ET develop the survey protocol and coordinated the lab activities. KEA analyzed the data and wrote the manuscript, HDB, RAD and RBK supervised the field activities (acquisition and collation of data). KES, MP and MH analyzed the data, EKD and KND provided technical validation of the protocol and BS contributed to the development of the manuscript. BS and YDA provided technical input and edited the manuscript. All authors approved the final version of the manuscript.
The authors acknowledge particularly Doctor Enyonam Tsolenyanu (national rotavirus AGE surveillance committee), Doctor Ndjao Akawulu (head doctor of the district of Tchaoudjo), the national Expanded Programme for Immunization and the Ministry of Health.
Copyright: © 2021 Foli Agbeko., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.