Dr Christian Chukwukere Ogoke1* and Dr Emeka Charles Nwolisa2
1
Paediatric Neurology Unit, Department of Paediatrics, King Fahad Central Hospital,
P.O. Box 204, Jazan 45196, Kingdom of Saudi Arabia.
2 Department of Paediatrics, Federal Medical Centre, P.M.B 1010, Owerri, Nigeria
*Corresponding Author: Christian Chukwukere Ogoke, Paediatric Neurology Unit, Department of Paediatrics, King Fahad Central Hospital, Abu Arish, Jazan, Saudi Arabia.
Received: August 08, 2020; Published: January 22, 2021
Citation: Christian Chukwukere Ogoke and Emeka Charles Nwolisa. “Gilles de la Tourette Syndrome - A Severe Case in an 8 Year-Old Nigerian Male”. Acta Scientific Paediatrics 4.2 (2021): 12-19.
Living with severe Gilles de la Tourette syndrome is physically and psychologically distressing for both the child and family and can impair quality of life and school attendance. Tourette syndrome is a chronic neurodevelopmental motor disorder manifesting with multiple motor and vocal tics. Tic disorders are common in school-aged children but there are few reports in Nigerian children and there is a dearth of physicians experienced in treating Tourette syndrome. This case report highlights the diagnosis and frustrations of management of severe Tourette syndrome in an 8 year-old male. More awareness of this neurodevelopmental disorder is needed in our locality.
Keywords: Tourette Syndrome; Tic Disorder; Management; Antipsychotics; Pimozide
Gilles de la Tourette syndrome (GTS) is a tic disorder with onset in childhood characterized by occurrence of multiple motor and vocal tics that wax and wane in frequency, lasting more than one year and not due to a medication/substance or another medical condition [1,2]. It remains the most complex and the most severe of the three tic disorders since it presents with more frequent, more complex, more severe motor and vocal tics and a greater number and severity of co-occurring neuropsychiatric conditions [1,3]. It is common among school-aged children, commoner in boys and occurs worldwide with a prevalence of 0.6 - 0.9% [4,5].
Tics are sudden, rapid, recurrent, stereotyped, non-rhythmic motor movements or vocalizations [2]. There are three tic disorders (provisional tic disorder, persistent/chronic motor/vocal tic disorder and Tourette disorder/syndrome) which are distinguished by type(s) of tics present and the duration of symptoms [1-3]. The exact cause of GTS and other tic disorders is unknown but like other neurodevelopmental disorders, multiple factors including genetic and environmental factors have been implicated [2,6]. Tourette disorder occurs more frequently among first-degree relatives which suggests a genetic component and the interplay between acute or chronic infections and other environmental factors in causing dopaminergic, serotonergic and glutamatergic neurotransmitter dysfunction and immunologic dysregulation has been reported by an earlier study [6]. Infections commonly associated with symptoms of tics are those caused by group A beta hemolytic Streptococcus (GAS), Mycoplasma pneumoniae and Borrelia burgdorferi [6]. Following streptococcal infections such as tonsillopharyngitis, the occurrence of tics, obsessive-compulsive disorder and increased motor hyperactivity or reduced fine motor coordination in a pre-pubertal child is referred to as Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections (PANDAS) [2,6]. Thus, PANDAS is a differential diagnosis of post streptococcal tics or acute exacerbation of tics following streptococcal pharyngitis or pyoderma.
The diagnosis of GTS is clinical and based on the occurrence of both motor and vocal tics and fulfilling the criteria stipulated in the DSM-5 as already stated above [1]. Laboratory tests and neuroimaging are therefore not needed to confirm classical presentations but may sometimes be employed to exclude secondary causes of tics such as post viral encephalitis, Huntington’s disease, stroke and insult to basal ganglia following head trauma [2,8]. Comprehensive evaluation of GTS involves an assessment of severity and evaluation for co-morbid disorders [2,9]. Useful symptom-rating scales include: Parent Tic Questionnaire, Tourettes’s Disorder Scale, Tourette syndrome severity scale (TSSS) and Yale Global Tic Severity Scale (YGTSS) with the latter being the most commonly used instrument for clinician-rated tic severity [2].Co-occurring psychiatric disorders include: Attention Deficit Hyperactivity Disorder (ADHD), Obsessive Compulsive disorder (OCD), mood disorders, Autism spectrum disorders (ASD), oppositional defiant disorder (ODD) and conduct disorders [2,9].Management of GTS of mild severity causing minimal, mild or no impairment or distress involves only psychoeducation of patient, family members and teachers [2].Severe GTS causing moderate, severe or marked functional impairment and distress would require additional measures such as class room accommodation or formal individualized education plan (IEP), behavioural treatment and pharmacotherapy [2]. The natural history of tic disorders is that of typical onset at 4 - 6 years of age, peak severity between ages 10 and 12 years and marked reduction of tics in two-thirds of individuals at age 18 - 20 years [2].
Patient C.U, an 8 year-old male who was referred to the Child Neurology Clinic of Mother Healthcare Hospital and Diagnostics, Owerri due to complaints of involuntary body movements x 7 months and involuntary vocalizations x 2 months - all prior to presentation 3 years 5 months ago. Involuntary movements were recurrent, sudden, rapid, purposeless jerks of parts of the body. The abnormal movements initially involved the face (facial twitching) then neck turning and shoulder shrugging and later progressed to a sequential jerking of the upper limbs. Other times, he frequently had eye blinking, eye darting, sudden backward jerking of his head, sudden arm flexion and extension and sudden forceful leg kicking.
These abnormal movements were exacerbated by stress, fatigue or tiredness or by being idle or unengaged. They were severe and disrupted his school activities. He tore his books while writing and distracted other pupils who frequently laughed at him and made him feel very uncomfortable. On some occasions, he had sustained bruises by hitting his hand/elbow on the wall or his mouth. Due to these, he was no longer willing to go to school. However, these movements usually abated during sleep.
About 5 months into the illness, he started making involuntary vocalizations that sounded like animal noises. He would have bouts of unnecessary coughing, throat clearing, sniffing and utterance of barking noises. Frequently, he repeated what other people said, copied other people’s gestures and had sudden changes in volume or pitch of speech. Later he started uttering obscene words and made complex vocal combinations.
There were no symptoms of inattention in class or hyperactivity/impulsivity and no history of obsessions or compulsions. There was no history of ingestion of substances such as cocaine and he was not on any medications prior to onset of above symptoms. Pregnancy, birth and early post natal history was unremarkable. There was no family history of tics, epilepsy or psychiatric disorders. His school performance was good. For the above complaints, he was seen by a medical officer who requested for brain MRI and planned to refer him to a psychiatrist. However, mother was verbally referred by a resident Paediatrician to our Child Neurology clinic.
The findings on physical examination at presentation were a fully conscious and alert boy with frequent involuntary, rapid, arrhythmic jerks of the limbs and vocalizations of animal-like noises. Initial diagnosis was provisional tic disorder since the duration of symptoms was less than one year. Initial management was nonpharmacologic as they were giving information about the diagnosis, its waxing and waning course, that it was not a psychotic illness or “madness”, that it can resolve with age without medications and also the likelihood of the multiple motor and phonic tics persisting beyond a year which will lead to a formal diagnosis of Tourette syndrome. The parents were reassured and counseled to largely ignore the tics and increase engagement in exercises. The class teacher was also to be educated on the child’s diagnosis so that the child will be better accommodated in class with less stigmatization.
Two weeks later, the child presented again to the clinic with complaints of increasing disruptive movements and severe tics that were disrupting both school and social activities. He was frequenting tearing his books in class while copying notes. He was further assessed and a formal assessment of tic severity was done using Yale Global Tic Severity Scale (YGTSS) from the Yale Child Study Center (October 1992 version) and the Parent Tic Questionnaire (PTQ). Screening for co-morbid conditions was done using the Vanderbilt ADHD Diagnostic Parent and Teacher rating scales. The following investigations were requested for: Full blood count (FBC), Liver Function test (LFT), serum electrolytes, urea and creatinine (SEUC), urinalysis, Thyroid function tests (T3, T4, TSH), throat swab for m/c/s, ASO titre and serum ferritin.
Assessment with YGTSS showed motor tic severity of 16/25, vocal tic severity of 16/25 and impairment of 30/50 (moderate) giving a total YGTSS of 62/100 (Figure 1). The Parent Tic Questionnaire (PTQ) indicated that he had 9 out of the 14 listed motor tics with many of them occurring hourly or daily with an intensity of 4 - 6/8 (obvious to others {≥ 4}, very noticeable or painful {≥ 6}) (Figure 2). Using the Vanderbilt ADHD Diagnostic Parent and Teacher rating scales, he did not fulfill the criteria for the diagnosis of ADHD and screened negative for Anxiety or depressive symptoms. However, by the Vanderbilt ADHD parent rating scale (but not the Teacher’s), he fulfilled the criteria for Oppositional defiant and conduct disorders while the performance section indicated no impairment (scored average or above average on all items).

Figure 1
Owing to the severity of his tics and unavailability of personnel trained in Comprehensive Behavioural Intervention for Tics (CBIT), he was commenced on tabs pimozide 1 mg daily after a prior ECG was reported by the Cardiologist as normal. Other requested investigations were not done due to financial constraints. Psychoeducation was further provided and the child taught how to build a competing response to tics when a premonitory urge is felt. On follow-up two weeks later, everybody was happy that the tics significantly reduced and he was attending school without tearing his books. About 6 weeks after commencement of tabs Pimozide, he required up to 2 mg daily to achieve good control especially during his examinations.
Patient defaulted with follow-ups but presented to the clinic after 6 months with complaints of exacerbation of both motor and vocal tics and painful swallowing. Patient had run out of Pimozide and the drug was no longer available in the whole country. Examination revealed significant discrete, multiple bilateral nontender cervical lymphadenopathy, erythematous pharynx and mildly enlarged tonsils bilaterally. Diagnoses of acute tonsillopharyngitis and tic exacerbation (flare-up) were made with PANDAS as differential diagnosis. Requested investigations were throat swab m/c/s and Full blood count. The following prescription was made: Tab Augmentin 457 mg BD x 10/7; to start tab risperidone 0.125 mg BD and increase to 0.25 mg BD. Throat swab m/c/s isolated Candida albicans - scanty growth.
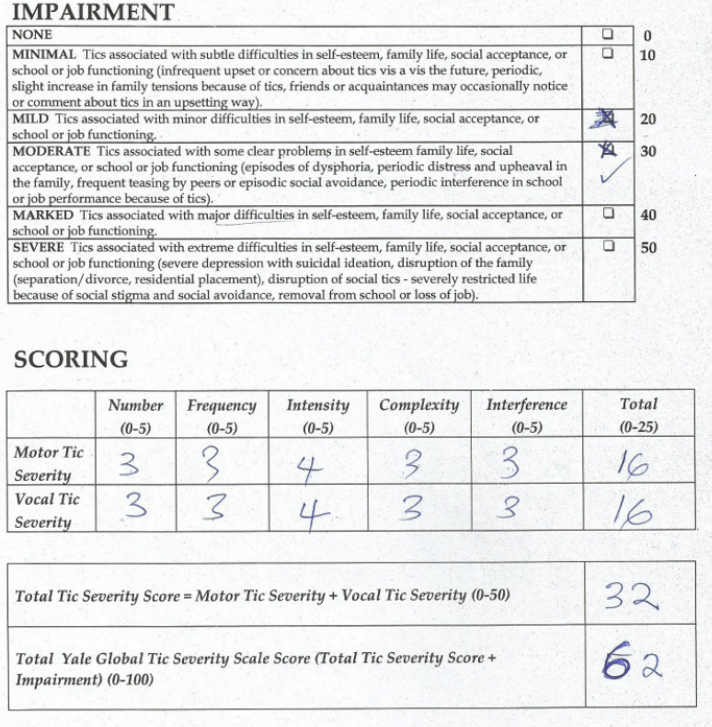
Figure 2
He was seen a month later with complaints of increasing severity and frequency of both motor and phonic tics despite increasing tab risperidone to 1.5 mg BD. Writing exams was severely impaired and he was noticed to have occasional involuntary smile which was a new tic. After a month of poor response to tab risperidone, medication was changed to tabs haloperidol 1.25 mg BD. With poor response, the dose of haloperidol was subsequently increased to 2.5 mg BD. Despite this multiple complex motor and vocal tics persisted and made it difficult for the child to go out the house. After some months, out of frustration, mother stopped all drugs and resorted to “fasting and prayers” and attending church deliverance services. Patient defaulted and was seen again a year later during a tic “off period”. He was said to have had a 2-month period of exacerbation which was the longest since onset of illness. Exacerbations were also noted to be more during the rainy season. On examination, he was neurologically normal with no noticeable motor or vocal tics. Diagnosis then was Tourette syndrome - off period. Patient was to try tab Aripiprazole 1.25 mg BD (to increase to 2.5 mg if necessary) if symptoms resurfaced. After this, he was lost to follow-up for two years. During this period, he consulted several other physicians in different parts of Nigeria due to persisting symptoms and poor response to available drugs. However, after a long search, he was able to procure tabs pimozide again. This was again efficacious and he continued it until he had occulogyric crisis and chest pain necessitating discontinuation of the drug. Presently, at 10 years of age, he is no longer on any drugs. Vocal tics have completely stopped while motor tics are still occurring but milder with much longer off-periods of up to 6 months.
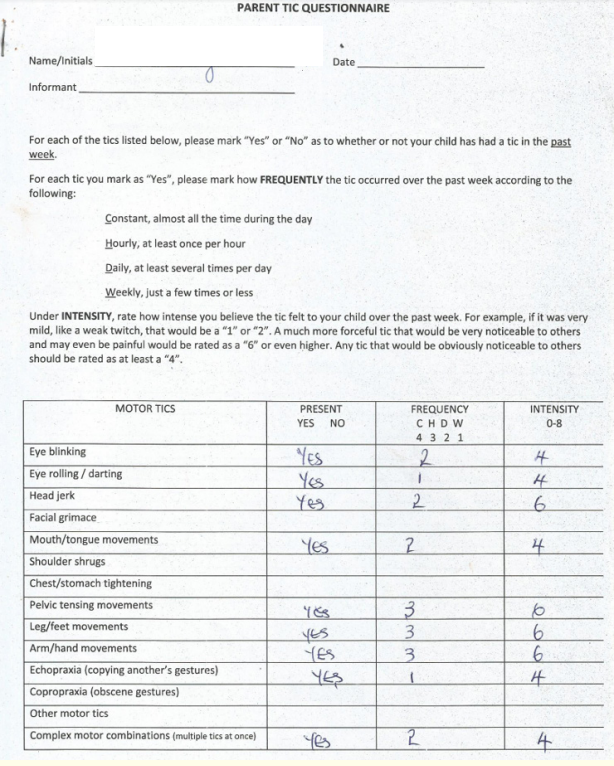
Figure 3
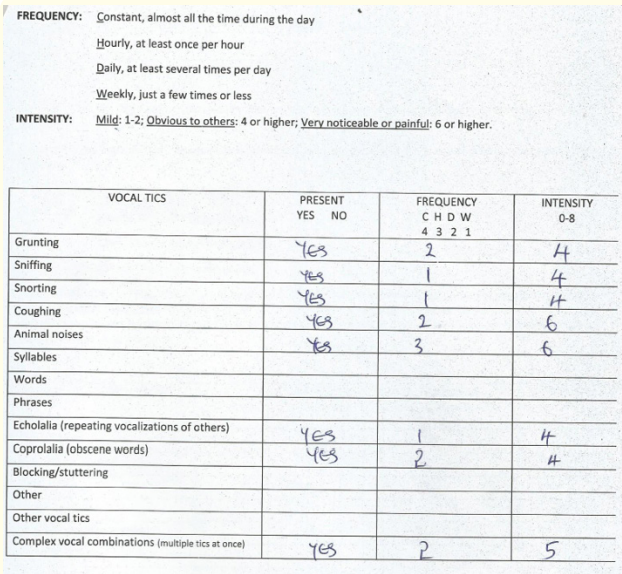
Figure 4

Figure 5

Figure 6
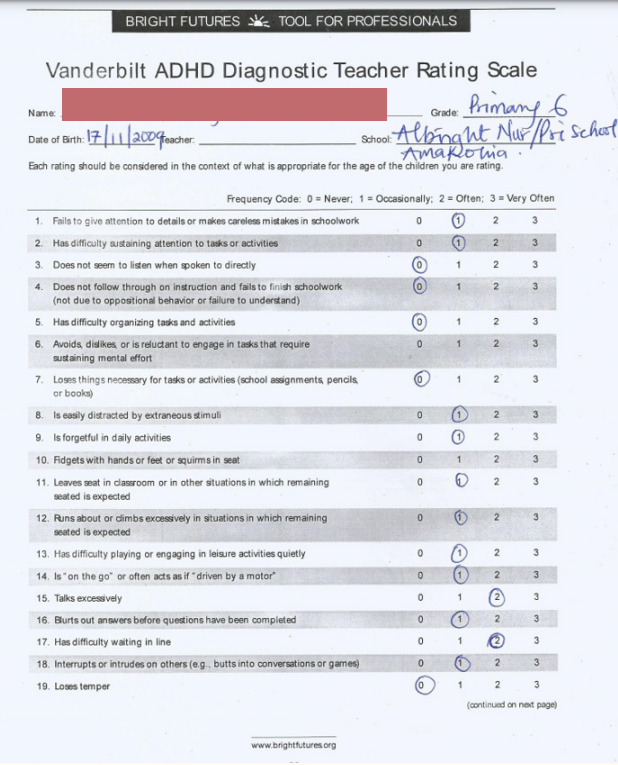
Figure 7
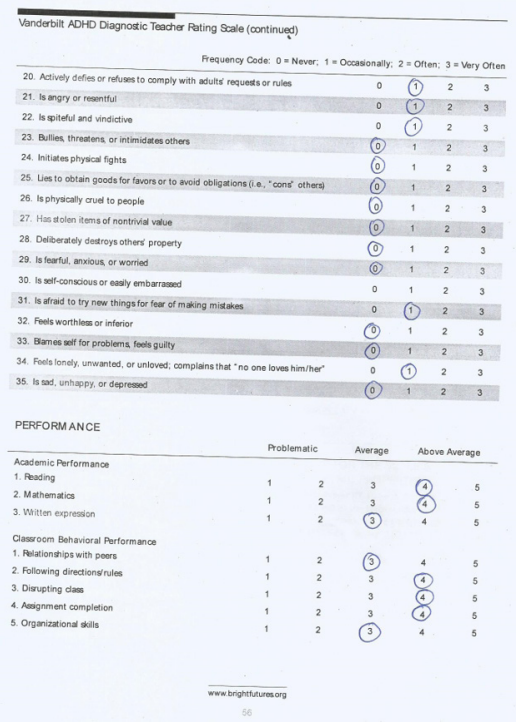
Figure 8

Figure 9
The case presented fulfilled the criteria stipulated by DSM-5 for diagnosis of Tourette syndrome [1]. Objective assessment of severity using two severity assessment scales for tics (one parent-rated scale - PTQ and one clinician-rated - YGTSS) classified this case as causing moderately severe functional impairment. The YGTSS is the most commonly used Tourette syndrome severity assessment scale and its reliability and validity have been reported by Murphy., et al. [10]. Evaluation for co-occurring psychiatric disorders is critical in patients with Tourette syndrome since these disorders may cause more functional impairment than the tics [2,9]. For co-morbidity assessment, the Vanderbilt ADHD Diagnostic Rating scale was used and this child did not have ADHD, anxiety or depression but screened positive for Oppositional Defiant and Conduct disorders. This child was not comprehensively evaluated for co-morbidities due to poor compliance with follow-up and financial constraints; the latter being the reason for failure to do most of the requested investigations. Multidisciplinary evaluation especially by a psychiatrist would have identified more co-morbidities in this child with moderate - severe Tourette syndrome.
A number of factors made the management of this child challenging: the poor health knowledge of people in our locality made psychoeducation difficult, the multiple and long defaults with follow-up clinic visits owing to a combination of factors including out-of-pocket payment for health services and the poor response to many neuroleptics that culminated in reversion to unorthodox medicare and spiritual healing. Additionally, the family had unrealistic expectations from pharmacotherapy despite few sessions of psychoeducation at the beginning. Furthermore, the lack of social support groups for children with Tourette syndrome in Nigeria denied this family of some emotional support and a feeling of “not being alone” that would have made them more patient with the clinician. The results of these for this child and his family were stigmatization, social isolation during flare-ups, physical and emotional distress, “doctor shopping” and interference with schooling and impaired quality of life.
It is clear to the neurologist that children with tics have variable response to different drugs and the aim of pharmacotherapy is to reduce the tics to a level where they are no longer causing functional impairment and not to totally eliminate them [2]. Failure to appreciate this could have resulted in the family having unrealistic expectations. However, the plight of a family with a child with multiple complex motor tics, echopraxia, echolalia and coprolalia is understandable. More so, the unavailability of the only efficacious drug, in this case - pimozide and the oculogyric crisis that necessitated its immediate cessation added to their frustration.
The exacerbation of tics in this child occurred mostly in the rainy season and coincides with the period of increased upper respiratory tract infections such as GAS - pharyngitis which is known to trigger or exacerbate tics [6]. It is surprising how this child with clinical features of acute tonsillopharyngitis during one of the tic flare-ups had a throat swab culture that yielded scanty growth of Candida albicans which is not a known common cause of sore throat. This may have been a contaminant. Research may also be needed in investigating the role of P. falciparum malaria in exacerbating tic symptoms since in our locality there is also a surge in the incidence of malaria during the rainy season. Finally, the frequency and severity of tics in this child have dwindled three years after onset and is expected to disappear in late adolescence. This conforms to the known natural history of Tourette syndrome [2].
This case report creates more awareness and highlights the distress of living with GTS and challenges of management of moderate-to-severe Tourette syndrome in a resource-poor setting.
The authors declare no competing interests.
Dr Christian C. Ogoke was involved in the evaluation, diagnosis and management of this patient. He conceived, designed and drafted this report. Dr Emeka C. Nwolisa revised the article critically for important intellectual content. Both authors read and approved the final manuscript.
We thank the staff of Mother Healthcare Hospital and Maternity.
Copyright: © 2021 Christian Chukwukere Ogoke and Emeka Charles Nwolisa. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.