Iliana Karagkouni1, Maria Sideridou2,3, Stavroula Stoupi1 and Georgios Efthimiou4*
1
Department of Dietetics, Metropolitan College, Athens, Greece
2
Department of Biomedical Sciences, Metropolitan College, Athens, Greece
3
Department of Nutrition and Dietetics, Hellenic Mediterranean University, Greece
4 Department of Biomedical and Forensic Sciences, Hardy Building, University of Hull,
United Kingdom
*Corresponding Author: Georgios Efthimiou, Department of Biomedical and Forensic Sciences, Hardy Building, University of Hull, United Kingdom.
Received: November 09, 2020; Published: December 29, 2020
Citation: Georgios Efthimiou., et al. “Association Between Breastfeeding and the Development of Childhood Diabetes Mellitus Type I (DM I): The Effect of Prolonged Exclusive Breastfeeding on Gut Microbiome and the Link with DM I”. Acta Scientific Paediatrics 4.1 (2021): 28-32.
The role of breastfeeding and dietary habits was studied in 10 children with DM I (case group) and compared with 10 healthy controls. Our results showed that the case group had significantly shorter exclusive breastfeeding duration compared with the control group (p = 0.006). An in silico comparative analysis of gut microbiota data from two recent studies was also performed in order to identify any specific bacterial genera potentially associated with DM I, due to prolonged breastfeeding. The Pasteurellaceae family, found in breast milk, was shown to have a significantly higher population in the intestine in the control group (p = 0.010). Overall, this preliminary study showed that exclusive breastfeeding duration is strongly associated with DM I. An association between breast milk microbiome and gut microbiome was also observed. This should encourage further research, aiming to examine both the effect of breastfeeding on gut microbiome and the possible links with DM I.
Keywords: Diabetes Mellitus Type I; Breastfeeding; Gut Microbiota
Diabetes mellitus type I (DM I) constitutes an autoimmune disease, which is characterized by pancreatic β-cells autoimmune destruction. Disease onset is commonly occurring in children, adolescents and individuals aged below 30 years old [1,2]. Over the last decades, incidence of DM I in children and adolescents has been increased worldwide. It is expected that in Europe the DM I prevalence among children aged below 15 years old will be increased by 70% by the year 2020 [3].
Several studies have been published to depict the relationship between dietary habits during early childhood and the risk of developing DM I [4,5]. According to studies, exclusive breastfeeding for the first 6 months of life is strongly associated with decreased risk of developing DM I during childhood [6,7]. Exclusive breastfeeding for more than 5 months and total breastfeeding for more than 7 or 9 months act protective against the development of DM I during childhood [7]. Another study demonstrated that breastfeeding, nicotinamide, zinc, and vitamins C, D, and E constitute possible protective factors against the development of DM I [8].
A strong relationship between maternal gut microbiota and offspring immune system is identified through several studies on mammals [9]. Microbiota antibodies and nutrients delivered by mother via milk, promote immune maturation of the child. Particularly, maternal gut microbiota enables expansion of gut innate immune cells, development of intestinal epithelial cells, mucus development and expression of antimicrobial peptides and antibodies secretion that prevent translocation of endogenous microbiota and hyper-reactivity to microorganism-derived compounds [9].
In the present study we aim to examine associations between breastfeeding and other dietary factors with DM I. Additionally we aim to identify possible associations between breast milk microbiome and children’s gut microbiome. This will help us to better understand the link between diet, gut microbiota and DM I.
The current study was conducted between October 2016 and February 2017 in a sample within Attika area, Greece. The study was approved by our Ethics Committee (Metropolitan College, Greece). All procedures and processes followed were in accordance with the ethical standards of the Declaration of Helsinki. Participants were 20 randomly selected children, aged 2-17 years old, who were born and are living within Attika area. From the total sample, 10 cases were diagnosed with DM I (case group) and 10 without (control group).
Breastfeeding duration was evaluated during infancy with a structured questionnaire. Using the Mediterranean Diet Quality Index (KIDMED) [10] assessed nutritional habits and the frequency of consumption of certain food groups.
All reported p-values were based on two - tailed hypotheses and compared to a significance level of 5%. SPSS version 19 (Statistical Package for Social Sciences, SPSS Inc, Chicago, IL, USA) will be used for the statistical analyses.
It was observed that children with DM I had statistically significant shorter exclusive breastfeeding duration compared to children without DM I (p = 0.006), with 50% of the participants (n = 5) in the control group to have exclusively breastfed for more than 5 months (Table 1). The majority of children with DM I were exclusively breastfed for 1-5 months (n = 6) while 33.3% of those (n = 3) were not breastfed at all. It was observed that the frequency of consumption of fruits, vegetables, red meat and its products, as well as sweets consumption did not differ significantly between the two study groups (p > 0.05).
Given that long-term exclusive breastfeeding may be protective against the disease onset [11,12], an in silico comparative analysis of genomic data from two Chinese studies [13,14] was conducted aiming to highlight associations between breast milk microbiota and gut microbiota in children with and without DM I. Extensive literature research using specific keywords on scientific online databases including PubMed, Scopus and Google Scholar, led to the identification of these two studies one describing the composition of breast milk from Chinese mothers and the other the composition of microbiota in Chinese DM I patients compared to healthy controls. It was important to ensure that the studies were carried out in the same country and city (Beijing), in order to eliminate factors such as genetic background, as well as differences in dietary and cultural habits, which could influence the composition of microbiome and its health impact.
Comparing the results, it was observed that Pasteurellaceae, which accounted for nearly 3% of the total bacterial population in breast milk (Figure 1), was shown to have a significantly higher population in the intestine in the control group (p = 0.010). A 2.6-fold increase in the population of Pasteurellaceae family was observed in healthy subjects (p = 0.010, Figure 1), with the genus Haemophilus to be a possible potential link (about to 2.6-fold higher abundance; p = 0.006, Figure 1) with DM I. No other genera in the Pasteurellaceae family showed differences between the DM I and health group.
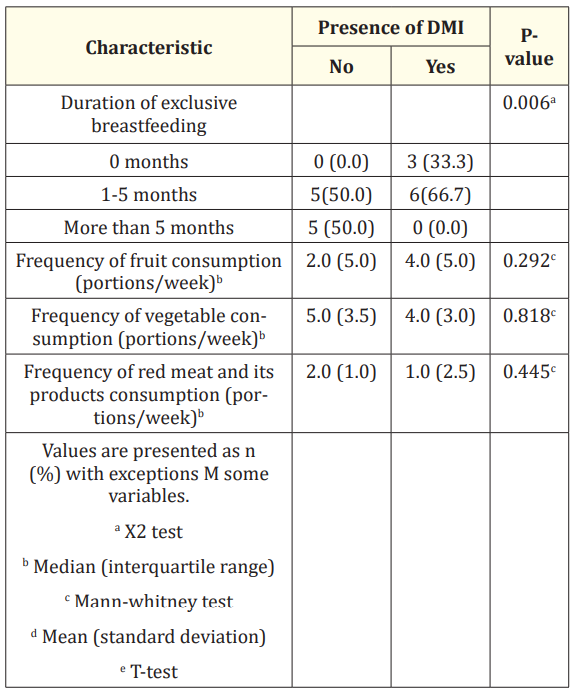
Table 1: Assessment of dietary intake for participants.
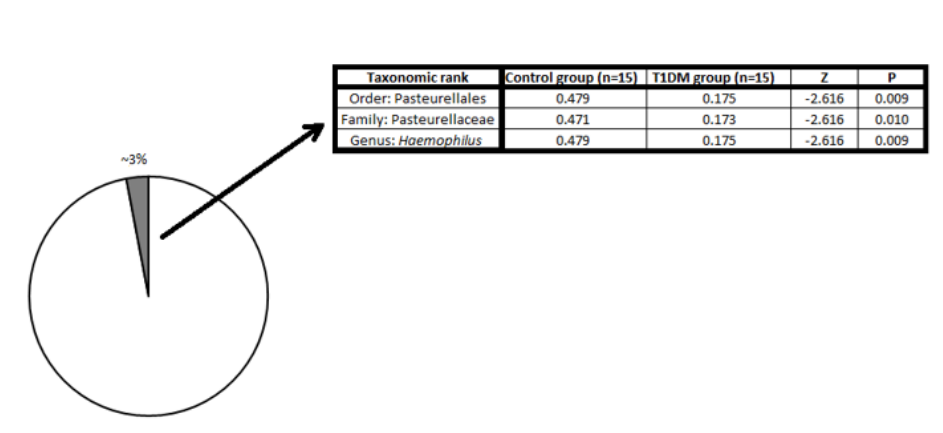
Figure 1: Comparison of the genomic data from two independent studies linking breast milk microbiome (left; (Sakwinska et al.)) with the bacterial composition of the gut microbiome in Type 1 Diabetes patients and healthy individuals (right; (Qi et al.)).
Our results described above are in agreement with previous studies [6,7,12]. It is suggested that exclusive breastfeeding for the first 6 months of life is highly preventive against DM I [10,15]. According to another study, children who have been exclusively breastfed for more than 5 months had 30% lower risk to develop DM I, compared with children who have been exclusively breastfed for only 2-6 weeks [16]. It is proposed that the protective role of breastfeeding against DM I development leads to increased levels of T-cells and decreased levels of inflammatory cytokines, such as interferon-γ, interleukin-4 and interleukin-10 [17]. Moreover, substantial content of insulin in human milk enhances gut maturation and minimises intestinal permeability to macromolecules, hence protection against the disease onset [18]. Other studies have observed and suggested that reduced exclusive breastfeeding duration did not show any significant increased risk in the advanced development of islet autoimmunity [9,19]. During the present study, it was observed that none of the children with DM I were breastfed for more than 5 months, whereas 50% of children without DM I were exclusively breastfed for more than 5 months. It is therefore suggested that prolonged exclusive breastfeeding duration (>5months) may be preventive against the risk of developing DMI during childhood. We also show that other dietary habits do not significantly affect DM I onset in childhood.
The in silico analysis performed in the current study, suggests that intestinal microbiota of children with DM I differ, compared to children without DM I. More specifically, the genus Haemophilus, a member of the Pasteurellaceae family which is abundant in breast milk, is present at higher proportion in controls’ gut microbiota, compared to DM I cases. These genera could be protective against DM I.
According to other studies, children with DM I have less diverse gut microbiota compared to healthy children, who have increased numbers of Bacteroidetes [20,21]. Specifically, children with DM I had larger populations of Clostridium, Bacteroides and Veillonella and significantly smaller populations of Lactobacillus, Bifidobacterium, the Blautia coccoides/Eubacterium rectale group and Prevotella, compared to children without DM I [22]. Another prospective study found that children in Finland and Colorado had a significantly lower bacterial variety, whereas children in Sweden and Washington had greater number of Bifidobacterium [23]. It must be considered that the diversity of gut microbiota is highly affected by geographical location. Therefore, we do not suggest that our study in Greece can be directly correlated with the two Chinese studies. However, in our attempt to explore which breast milk components could affect DM I development, we propose some very interesting candidates.
Overall, this preliminary study showed that exclusive breastfeeding duration is strongly associated with DM I, in Greek participants. An association between breast milk microbiome and gut microbiome was also observed. Further investigation of bacterial population, immune maturation and inflammation is needed, in order to investigate thoroughly the role of gut microbiota in autoimmune development. Our original findings highlight the importance of breastfeeding for human health and could lead to new probioticbased strategies for dealing with DM I in the near future.
This project was funded by Metropolitan College. There are not any conflicts of interest.
We would like to acknowledge the efforts of the volunteers` and their families to participate in this study and thank them.
Copyright: © 2021 Georgios Efthimiou., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.