Liliana Sá1*, Cátia Leitão2, Jorge Romariz3, Herculano Costa3, Fátima Praça3 and Cláudia Pedrosa3
1Pediatrics and Neonatology Department, Centro Hospitalar de Entre-o-Douro e Vouga, Portugal
2Pediatrics Department, Centro Hospitalar de Vila Nova de Gaia/Espinho, Portugal
3Pediatrics Department, Pediatric Immunoallergology and Pulmonology Unit, Centro Hospitalar de Vila Nova de Gaia/Espinho, Portugal
*Corresponding Author: Liliana Sá, Department of Pediatrics and Neonatology, Centro Hospitalar de Entre Douro e Vouga, Portugal.
Received: October 28, 2020; Published: November 30, 2020
Citation: Liliana Sa., et al. “Fixed Drug Eruption Induced by Paracetamol - A Case Report”. Acta Scientific Paediatrics 3.12 (2020):27-29.
Fixed drug eruption can manifest as one or multiple lesions and may be caused by various types of drugs. Paracetamol is one of the common drugs prescribed as analgesic–antipyretic agent in all age group of patients. Its severity spectrum is highly heterogeneous. Fixed drug eruption is a well-reported, but uncommon side-effect of paracetamol, usually the classic, pigmenting type most commonly found in children and adolescents. The authors report the case of an adolescent with this rare entity, warning of its clinical suspicion in cases of recurrent skin drug reactions always in the same location.
Keywords: Drug Eruption; Paracetamol; Antipyretic; Fixed Drug Eruption; Adverse Drug Reaction
FDE: Fixed Drug Eruption; OTC: Over the Counter
Adverse reactions to drugs can be defined as any response elicited by a given drug that is harmful, unintentional and occurs at the doses used by individuals for prophylaxis, diagnosis and treatment of diseases [1]. Reactions involving the skin, known as pharmacodermy, are among the most common and can have many clinical appearances – from solitary lesions up until generalized rashes [2]. FDE is a distinctive type of cutaneous drug reaction that characteristically recurs in the same locations upon re-exposure to the offending drug. Is frequently misdiagnosed, leading to recurrent eruptions when the offending drug is readministered. It occurs in both genders and in all age groups. At pediatric patients, account for 14 to 22% of cutaneous drug reactions. Antibacterial sulfonamides, antibiotics, nonsteroidal anti-inflammatory drugs, analgesics and hypnotics are the most frequent causes [3]. Paracetamol is a readily available OTC antipyretic. Despite its widespread use, adverse reactions are unusual at therapeutic dose.
A 16-year-old male patient, with personal history of asthma and rhinitis, was referred to a pediatric allergology consultation due to a history of three episodes of right lower lip edema, beginning 10 to 30 minutes after taking paracetamol, with spontaneous resolution in less than 24 hours. The patient had no other complaints and no other relevant past medical history. He underwent paracetamol prick and intradermal skin tests that were negative and was then submitted to oral provocation challenge. During the test, three hours after onset, an erythematous lesion and circumscribed edema in the right lower lip mucosa appeared, with pruritus and local dysesthesia (Figure 1 and 2). There were no other alterations on physical examination. Spontaneous resolution of lip lesion in less than half an hour. Four hours after the start of the test, a new dose of paracetamol was administered, with reappearance of the lesion with similar characteristics and symptoms, also with spontaneous resolution.
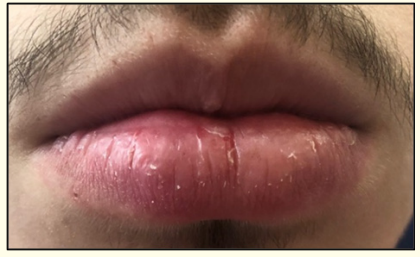
Figure 1: Right lower lip edema.
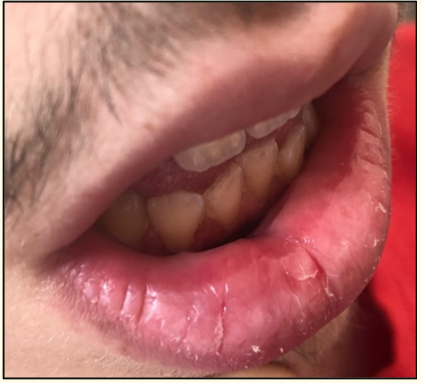
Figure 2: Violet and erythematous lesion in the right lower lip mucosa.
Paracetamol induced FDE is reported in the literature in less than 1.5% of all cases of FDE [4]. The offending drug is thought to function as a hapten that preferentially binds to basal keratinocytes, thereby releasing lymphokines and antibodies thus damaging the basal cell layer [5].
The exact pathogenesis of this type of drug reaction is unknown. According to one hypothesis, FDE is classified as a type IVc immunologic reaction because of latent cytotoxic CD-8 positive T cells in the lesions, which may become reactivated. There is also an association with HLA class I antigens, suggesting that there may be a genetic predisposition to these reactions [6].
About the clinical findings, the skin lesion is usually a dusky erythematous macule and is mostly found on the extremities, lips, genitalia and perianal areas; however any skin or mucosal surface can be involved. A burning sensation is one of the associated symptoms. The lesions may be present in multiple numbers an can arise with central vesicles and bullae, particularly after the repeated use of the drug [7]. Acute lesions generally appear 30 minutes to 8 hours after drug administration, but can occur up to two weeks. After discontinuation of the offending drug, lesions resolve spontaneously in 7 to 10 days, leaving a persistent gray/brown or slate gray postinflammatory hyperpigmentation. Upon reexposure lesions typically recur in the same site, but new lesions may develop elsewhere. After one or more localized eruptions, FDE rarely may evolve into a bullous generalized form mimicking Stevens-Johnson syndrome/toxic epidermal necrolysis [8].
The diagnosis of FDE is based in typical clinical findings and history of drug exposure. Histologic examination of a skin biopsy is helpful in establishing the diagnosis in cases with unusual presentation [3]. Oral challenge and skin patch can be performed to identify the culprit drug when history is unclear or multiple medications are suspected. In most cases, oral challenge is the gold standard diagnostic test reproducing the conditions of exposure. It is considered relatively safe because the cutaneous response is located and the risk of a severe reaction is low. However, it is contraindicated in patients with a history of generalized FDE [9].
In this case, the patient suffered from FDE due to paracetamol for the first time in his life though there was history of taking paracetamol many times earlier without any such occurrence. It was probably the gradual accumulation of memory T cells over a certain period responsible for such new onset FDEs occurring for the first time even though there was history of drug exposure many times in the past.
Discontinuation of the offending drug is the most important part of management of FDE. After drug discontinuation, lesions resolve without treatment in a few days leaving postinflammatory hyperpigmentation. Patients should be advised to avoid the offending drug and chemically related drugs and be provided with a written list of the generic and brand names of the culprit drug and cross-reactive drugs [3].
In countries where many drugs are available OTC which can produce serious as well as non-serious adverse reactions, it becomes very difficult to identify the exact etiological agent when such a reaction occurs. Even previous history of uneventful administration of any commonly used OTC drug does not make it safe for future unadvised administration.
The authors have no conflicts of interest to declare.
This work has not received any contribution, grant or scholarship.
Copyright: © 2020 Liliana Sá., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.