Dixon-Umo OT1*, Ikpeme EE2 and Kan KM3
1Senior Lecturer and Honorary Chief Consultant Paediatrician, Department of Paediatrics, University of Uyo/University of Uyo Teaching Hospital, Uyo, Nigeria
2Professor of Paediatrics and Honorary Chief Consultant Paediatrician, Department of Paediatrics, University of Uyo/University of Uyo Teaching Hospital, Uyo, Nigeria
3Fellow West African College of Physicians and Senior Registrar, Department of Paediatrics, University of Uyo Teaching Hospital, Uyo, Nigeria
*Corresponding Author: Dixon-Umo OT, Senior Lecturer and Honorary Chief Consultant Paediatrician, Department of Paediatrics, University of Uyo/University of Uyo Teaching Hospital, Uyo, Nigeria.
Received: June 25, 2020; Published: August 17, 2020
Citation: Dixon-Umo OT., et al. “Urinary Tract Infection in Infants and Pre-school Children at a Tertiary Hospital in Uyo, Nigeria: The Prevalence, Clinical and Bacteriological Profiles”. Acta Scientific Paediatrics 3.9 (2020):02-09.
Background: Urinary tract infection (UTI) is a common cause of paediatric febrile illnesses and pyelonephritis could be complicated with renal scarring, predisposing to hypertension and chronic kidney disease in later life. High index of suspicion is essential for early diagnosis and prompt treatment.
Aim: We set out to determine the prevalence of UTI; to evaluate its clinical presentations and bacteriologic profiles among febrile infants and pre-schoolers.
Methods: A prospective cross-sectional study of 165 febrile children aged one to sixty months, using urinalysis, microscopy, culture and sensitivity was done on clean catch/midstream urine samples. Data was analyzed using Statistical Package Social Sciences version 22.0. Statistical significance value was p < 0.05.
Results: Of the 165 children, 23 (13 females and 10 males) had UTI (isolation of a single pathogen 105 CFU) and Staphylococcus aureus, an unusual pathogen, was the commonest isolates (34.8%). Escherichia coli (30.4%), Proteus mirabilis (26.1%) and Citrobacter freundii (8.7%) were also isolated. Vomiting was the only significant (P = 0.04) symptom among majority of subjects with Enterobacteriaceae UTI. The area under the curve (AUC) for WBC was 0.700 being higher than that of nitrite, 0.504 and leucocyte esterase, 0.467 respectively, p = 0.002.
Conclusion: The prevalence of UTI among febrile infants and pre-school children was as high as 13.9% with Staphylococcus aureus being the commonest aetiologic agent. Vomiting was a significant clinical association. Urinalysis showed a low sensitivity for nitrite and leucocyte esterase depicting its low sensitivity and usefulness as a diagnostic test.
Keywords: Clinical Presentations; Bacteriological Profiles; Prevalence; UTI; Febrile Children
Urinary tract infection (UTI) as one of the common causes of fever [1,2] in children accounts for a large proportion of paediatric morbidity and hospitalisations [3,4]. Its complications including renal scars can become an eventual cause of non-essential hyper tension and chronic kidney disease [2]. Paediatric UTI has a global prevalence of 2 - 20% [5-13], occurring in all races but with some variations in gender and a higher presentation in those less than five years of age [7,14,15].
Fever is often the most common symptom of UTI in infants and young children but several other non-specific symptoms like vomiting, feeding problems, failure to thrive, diarrhoea, irritability, abdominal pain and offensive urine also occur [15]. Sometimes acute pyelonephritis may occur with fever as the only presenting symptom and though it may go unnoticed, it is still capable of causing renal injury and subsequent renal scaring, hypertension and chronic kidney disease [14].
Vomiting has also been noted as another very common nonspecific symptom among young children with UTI. Earlier reports from Nigeria by Ibeneme., et al. [9] in Enugu, southern Nigeria, showed that vomiting and abdominal pains in febrile children with UTI were important non-specific presentations. Also, Rabasa and Gofama [12] in Maiduguri, northern Nigeria, found that vomiting was the commonest symptom of paediatric UTI.
Although more specific symptoms of UTI including dysuria (pain on urination), suprapubic pain, flank pain, urinary frequency, haematuria and urinary incontinence, are more difficult to obtain in young children [14,16,17], Indirect evidence from parental history of the child’s reluctance to urinate, excessive crying on micturition of behaviour suggestive of abdominal pain may depict dysuria [16,17].
Urine culture is the gold standard for UTI diagnosis while urinalysis is a useful screening test [14,15,17].
Bacteria are usually the common aetiologic agents of UTI but viruses and fungi are also implicated, as well as parasitic cause like trichomoniasis in sexually assaulted children [14] Of the bacterial causes, the Enterobacteriaceae such as Escherichia coli, Klebsiella spp, Proteus spp, Pseudomonas spp and Citrobacter spp are predominant. Other organisms like Staphylococcus aureus and Staphylococcus epidermidis, Morganella morganii, group B Streptococcus have also been implicated [14]. E. coli is the most widely isolated pathogen in most UTI in infants and children [14].
The prevalence of UTI among infants and pre-school age children as well as the implicating organisms have not yet been documented in our health facility, hence the need to determine the prevalence of this common infection among febrile under five children. The study was also set to highlight the common clinical presentations and to identify the bacteriological agents and to note the antimicrobial sensitivity to aid in more decisive treatment of under five children presenting for care at the paediatric infectious diseases unit of our developing teaching hospital.
A prospective cross-sectional study was done from June to September 2017. It involved children aged one month to 60 months who presented with axillary temperature greater than 37.40C [18] at the children’s outpatient clinic and children’s emergency unit of the University of Uyo Teaching Hospital, Uyo. Children who had received antibiotic for 2 weeks prior to or at onset of fever were excluded. Other exclusion criteria were: predisposing conditions of UTI such as those diagnosed with anatomical urinary tract abnormalities such as posterior urethral valves, presence of chronic diseases like human immunodeficiency virus infection/acquired immune deficiency syndrome (HIV/AIDS), severe protein energy malnutrition and sickle cell anaemia.
Ethical approval was obtained from the University of Uyo Teaching Hospital’s Institutional Health Research Ethical Committee (IHREC). Informed consent was obtained from parents/caregivers of all the children.
A questionnaire was filled for all children who met the inclusion criteria and their demographic details, relevant clinical history and examination were obtained and recorded on a proforma.
Urine collection was on the spot by midstream/clean catch [19,20] for all children into 2 sterile containers. This was achieved by giving the children fluid (breastmilk or water for children older than 6 months) 30 minutes to 1 hour before sample collection while they were awaiting consultation. For preverbal children, the external genitalia were cleaned with sterile gauze soaked in clean water then dried with sterile dry gauze. An assistant held the child parting the legs wide apart while the lead investigator stimulated the bladder by applying slight pressure over the suprapubic region by frequent gentle tapping over the suprapubic region with the fingers until the child voided urine. As the child began to void, a clean catch specimen of the urine was collected into 2 sterile containers, at least 2 mls in each container, while maintaining strict asepsis. For the verbal children, the genitals were cleaned with wet sterile gauze and dried with dry sterile gauze. The child was asked to void urine and the mid urine stream was collected into two sterile taken to the microbiology laboratory within 30 minutes of collection and evaluated immediately for microscopy and culture. Specimens were stored in a refrigerator at 40C when sample could not be assayed the same day.
Urinalysis for nitrite and leucocyte esterase using Combi-11 strip (TRUE SCREEN COMBI 11A by LIFESANE BIOTECH, U.S.A) was done for the urine specimens and recorded accordingly. Urine microscopy was done on the samples. Semi-quantitative assay by a calibrated sterile loop of 0.01 ml of urine was plaited on Cystein Lactose Electrolyte Deficient (CLED) agar and then incubated at 370C for 24 hours. Growth of 105 colony forming units per ml of urine was regarded as UTI. Isolates were identified by standard methods elaborating colony morphology, Gram stain, oxidase and catalase test.
Data was recorded in the participant’s worksheet and transferred into Microsoft Excel 2016 (Microsoft Corporation, USA). Data analysis was with statistical package for the social sciences (SPSS) version 22. Information obtained was presented in tables and figures. The mean age of subjects and the prevalence of UTI were determined. Categorical data were represented as frequencies and percentages. Chi square was used to test for associations and significant P–value was set at less than 0.05.
The study recruited 165 consecutive eligible children of which 88 (53.3%) were males, with a male to female ratio of 1.14: 1. The mean age of participants was 23.56 ± 19.95 months with a range of one to 60 months.
Twenty-three subjects had positive culture results with significant bacteriuria of 105 giving the UTI prevalence of 13.9% as illustrated in figure 1.

Figure 1: Pie chart showing the proportion of children with UTI.
Table 1 shows the distribution of clinical symptoms among the study population. Vomiting was the next commonest symptom coming occurring in 45 (27.3%) subjects. None of the subjects had specific urinary symptoms such as dysuria, frequency or loin pain.
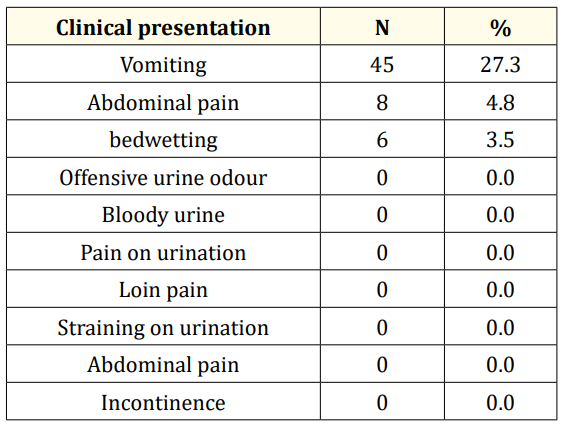
Table 1: Clinical features of the study subjects.
Table 2 shows the values of AUC obtained from ROC curve for nitrite, leucocyte esterase and white cell count (WBC) per high power field (hpf). The result obtained shows that area under the curve of WBC is 0.700 which is higher than that of nitrite and leucocyte esterase of 0.504 and 0.467 respectively. This difference in AUC is statistically significant at 0.002 (< 0.05). This indicates that WBC may be regarded as an important indicator of the presence of UTI. The sensitivity and specificity of nitrite test were 43.0% and 98.0%; while that of leucocyte esterase tests were 46.0% and 97.0% and for WBC/hpf they were 47% and 77% respectively.
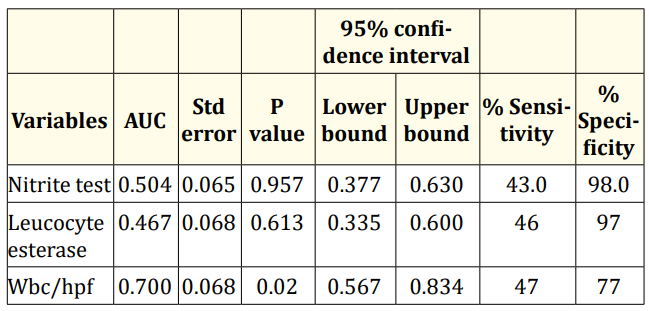
Table 2: Area under curve for urine nitrite test, leucocyte esterase test and white blood cells.
Considering bacterial isolates from urine culture, Staphylococcus aureus was the commonest isolated bacterium (34.8%) followed by the Gram negative organisms: Escherichia coli (30.4%), Proteus mirabilis (26.1%) and Citrobacter freundii (8.7%).
Table 3 shows the comparison of culture proven UTI with clinical features. Vomiting was the only significant symptom noted in most children with Enterobacteriaceae UTI. Other symptoms were not significantly associated with aetiologic pathogen. Only one child with E. coli UTI had a positive nitrite test but this was not statistically significant.
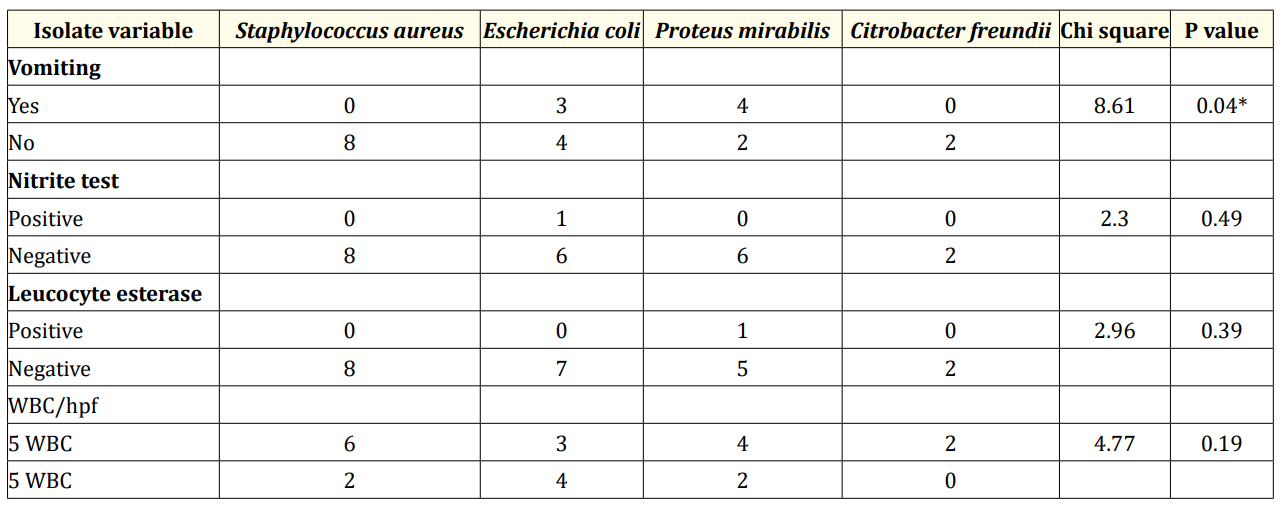
Table 3: Comparison of culture proven UTI with clinical features, urinalysis result and white cell count. *= significant p value.
Table 4 shows that the highest occurrence of UTI was 39.1% observed in male infant. Citrobacter infection occurred only in male infants who did not have E. coli infection. Next to infants, were those between 13 - 24 months old and 49 - 60 months of age each with 21.7% of confirmed UTI which occurred more in females. Staphylococcus aureus affected all age groups equally but was more prevalent in the males, but Escherichia coli was found to predominate in females. Proteus mirabilis occurred twice as much in females as in males. There was no statistically significant difference among the age groups (p = 0.41) but gender differences were significant at p = 0.009.
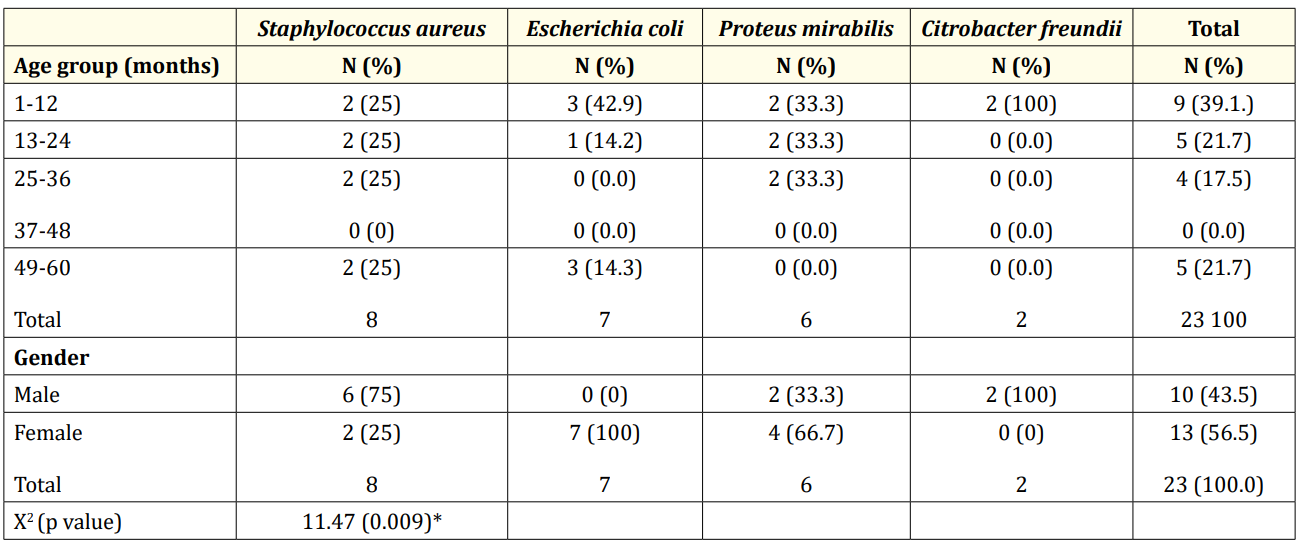
Table 4: Distribution of Isolates among the age groups and gender.
The prevalence of UTI of 13.9% in this study is comparable to other African studies with reports of 13.7% [12] from Maiduguri, Nigeria; 14% [17] from South Africa and 16.8% [8] from Tanzania. Lower prevalence rates between 9.0% - 11.9% [9-11] have been reported by several authors in different parts of Africa and their study populations were also that of febrile under-fives. This explains the similarity of their prevalence rates to what was obtained here because children below the age of five years are more prone to infections including UTI.
Much lower prevalence rates of 3.3% [21], 4.0% [5] and 5.3% [22] have also been reported by authors in the resource-rich countries of United States of America and United Kingdom. This is expectedly so as infection rates are lower due to the better sanitary conditions and ready access to standardized health care made possible by health insurance coverage. In addition, Shaw., et al. [21] excluded febrile children with a focus of infection and this could have accounted for the low prevalence as UTI could possibly be a co-morbidity of other paediatric febrile illnesses. To satisfy a high index of suspicion, every febrile child should still be investigated for UTI, despite an identifiable focus of infection.
Also, the inclusion criteria by Shaw., et al. [21] and Hoberman., et al. [5] were febrile children with temperature greater than 38.50C whereas this study used a lower threshold of fever at an axillary temperature greater than or equal to 37.50C [18]. Using a higher threshold for could have excluded children with UTI with lower temperature readings as used in those studies from the resource rich countries. Similarly, Muoneke [13] in Abakaliki reported a low prevalence of 3% compared to other African studies. This marked difference could have been due to the peculiarities of a retrospective study with a possibility of missed data, misplaced results, and/ or inadvertent under diagnosis of UTI in children with non-specific symptoms.
Deviating from the index findings, were higher prevalence rates of UTI reported by other researchers in the resource limited of south western Nigeria (21.4%) [3], Uganda (26.8%) [23] and India (28.3%) [24]. The Indian study included high risk surgical patients with conditions like posterior urethral valve and vesico-ureteric reflux which this study excluded while the high prevalence rate in Uganda could have been because of the use samples of bag urine which is fraught with associated higher rates of microbial contamination [20].
UTI is more prevalent in early childhood especially in those less than 24 months as was reported in this study population [2,8]. Majority of the children were less than 12 months of age. This agrees with the findings of Muoneke., et al. [13], Shaw., et al. [21], Fredrick., et al. [8] and Musa-Aisien., et al. [10] who also noted higher occurrence rates of UTI in children less than 24 months of age. This is thought to be due to their inability to control the flow of urine predisposing them to incomplete bladder voiding and thus leading to VUR [14] Also the possible contribution of an immune system that is not yet fully developed may be a contributory factor.
Male infants were twice more likely to have UTI and this corroborates with the findings of Taneja., et al. [24] who also found paediatric UTI to be more common in males infants. Contrastingly, the study from Enugu [9] showed no sex predilection among infants with UTI. The differing findings are not readily explainable. It has been postulated that male infants have greater presentations of genito-urinary tract abnormalities majority of which are congenital, which predisposes them to UTI.
Beyond infancy, the female to male ratio was 2.5:1, a comparable finding to that from Ibeneme., et al. [9] in which females were three times more likely to be infected with UTI. Similar observations were also noted by Aiyegoro., et al. [7] and Musa- Assien., et al. [10]. This is attributed to the short urethra and its close proximity to the anal orifice. This anatomical proximity promotes the spread of faecal pathogens from the anal orifice into the urethra [14] and onward ascension into the urinary bladder and possibly to other sites of within the urinary tract.
Vomiting, an important non-specific symptom among children with UTI, was the commonest symptom, highlighting the importance of non-specific symptomatology of UTI in the under-five population and this corroborates the report of Rabasa and Gofama [12] in Maiduguri where vomiting was also commonest. Additionally, Ibeneme., et al. [9] in Enugu reported that vomiting though in combination with abdominal pains was the commonest clinical presentation of febrile children with UTI. This non- specific clinical presentation of UTI in under-fives contrasts with the findings of specific urinary symptoms of dysuria and urinary frequency among children less than 5 years of age by Fredrick., et al. [8] in Tanzania. The observed difference may be because majority of those with UTI in this study were non -verbal children below 24 months of age.
Urinalysis showed low sensitivity parameters for urine nitrite and leucocyte esterase tests which was comparable with the low values reported by Tsai., et al. [25] and Senthilkumar., et al. [26] this may have been because urine collection was on the spot, thus not allowing enough time for nitrate conversion to occur. The predominance of Gram-positive organisms in this study may have contributed to the low sensitivity obtained as certain Gram-positive organisms lack the nitrate reductase enzyme to convert nitrate to nitrite [27]. On the whole, urinalysis as a lone test for diagnosis of UTI would likely have resulted in remarkable missed diagnosis especially in this age range.
Staphylococcus aureus, a Gram-positive pathogen, was the commonest pathogen isolated followed by E. coli, in accordance with findings by different authors in Benin [4,10,28]. This similarity could be as a result of the index study area being in the same Nigerian geopolitical zone suggesting that Staphylococcus aureus maybe uropathogen in febrile children seen at hospital in this region. Also, the occurrence of this pathogen may be associated with hematogenous spread of infection. Escherichia coli, a common uropathogen from studies carried out in both rich and resource-limited countries [5,8,9,14,21] was next in predominance. Staphylococcus aureus UTI of up to 22.7% was earlier reported by Ibeneme., et al. [9] in a southern Nigerian hospital-based study. Shaw., et al. [21] also reported a small proportion of Staphylococcus aureus accounting for UTI pathogens in their study population from United States of America.
Taneja., et al. [24] in India found E. coli (47.1%) and Klebsiella pneumoniae (14.5%) as the commonest pathogens of UTI among less prevailing organisms like Citrobacter, Proteus, candida, Pseudomonas aeruginosa and Staphylococcus aureus while Masika., et al.[23] in Kenya found E.coli the aetiologic uropathogen in 64.5% of children less than 5 years, followed by Staphylococcus aureus (12.9%).
In contrast, Ocokoru., et al. [23] in Uganda noted Proteus spp as the most prevalent aetiological agent of UTI among febrile under - five children, accounting for 39.5% followed by Escherichia coli (32.1%) and Staphylococcus aureus (14.8%).
Citrobacter freundii, a common causative organism among male infants in this study though uncommon in other studies [9,21], was comparable to the findings of Taneja., et al. [24] who also noted Citrobacter spp as a cause of UTI in their study population. The reason for this is not well understood, perhaps, being an enteric organism, it could ascend the urethra and cause UTI in this age group.
Febrile infants and pre-school children had a high UTI prevalence of 13.9% and vomiting was a significantly associated nonspecific symptom. Male predominance occurred during infancy but females predominated in the post infancy age period. The common uropathogens were Staphylococcus aureus, Escherichia coli, Proteus mirabilis and Citrobacter freundii in that descending order. Urinalysis showed low sensitivity for diagnosis of UTI and universal urine culture is recommended for diagnosis of UTI in febrile children
Copyright: © 2020 Dixon-Umo OT., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.