Isabel Pizarro Veas1*, Domingo Roman-Silva1 and Carlos Solar Barrios2
1Laboratory of Bioinorganic and Environmental Analytical Chemistry, Department of Chemistry, Faculty of Basic Sciences, Universidad de Antofagasta, Av. Universidad de Antofagasta, Antofagasta, Chile
2Unity of Cardiovascular Surgery, Clínica Antofagasta, Manuel Antonio Matta,Antofagasta, Chile
*Corresponding Author: Isabel Pizarro Veas, Laboratory of Bioinorganic and Environmental Analytical Chemistry, Department of Chemistry, Faculty of Basic Sciences, Universidad de Antofagasta, Av. Universidad de Antofagasta, Antofagasta, Chile.
Received: April 06, 2020; Published: June 16, 2020
Citation: Isabel Pizarro Veas., et al. “Arsenic Effects in Cardiovascular Diseases of People Living in Contaminated Areas of Chile”. Acta Scientific Paediatrics 3.7 (2020):46-53.
The concentration levels of As in the Chilean II Region of Antofagasta produces non cancer health outcomes such as cardiovascular diseases and in last term heart attack. On this study, the determination of total As content and main inorganic and organoarsenic species found in three heart tissues (auricle, mammary artery and fat) and the saphene vein of people living in the Chilean II Region, suffering coronary thrombosis has been carried out. Comparison with similar tissues of patients from other non-contaminated areas has been undertaken. The auricle and in less extend the saphene vein support the higher As concentration (mean values of 7.7 and 2.5 µg g-1, respectively), being As(III) the predominant species. Methylation towards MMA and DMA is not a favoured mechanism.
The presence of high total As and high As(III) species content in the auricle and saphene vein of more contaminated people, the damage found in the saphene vein tissue and the global characteristics of the people under study in which the As stigmas are present in all of them, suggests that As could be involved in the cardiovascular diseases.
Keywords: Arsenic Exposure; Cardiovascular Tissues; Arsenic Speciation; Total Arsenic Concentration: Medical Geology; Conditional Variables; Arsenic in Contaminated Cardiovascular Tissues
The coastal - Andean Mountain - Upper Highlands Ecosystem of the II Region of Chile is an important area of the Atacama Desert; of which the River Loa basin is a part. This river is an aquatic desert ecosystem, and the source of drinking water for the cities in the II Region of Antofagasta in Chile. However, this particular ecosystem suffers from the chronic impact of endogenous arsenic due to volcanism in the area, and anthropogenic delivery of arsenic and other heavy metal due to mining activity [1], which transports trace elements more rapidly into ecosystem in comparison to the normal geological process, thus spreading the heavy metals to human beings, through the biogeochemical cycles [2].
It is worldwide known that the Chilean II Region has a heavy concentration of mining activity, related to the copper production and non-metallic salts, producing more than two million tons of copper annually [3] and the well-known “nitrato de Chile” from the potassium nitrate mines named “salitreras”. The Antofagasta city, in the west - centre of the Region, centralizes the social services and in it outskirts is located some industries related mining. So, the two principal sources of the arsenic impact on the human beings are the As associated to the mineral process which produces arsenic contamination in the zone affecting people directly in some work places, and the arsenic contamination problem from the main water supply. During the period 1950 - 1970, the medium As con[4]. After installation of an As removal plants, the arsenic concentration in drinking water dramatically decreased up to the recommended maximum level of about 50 µg L-1, but fluctuations around this value gives a relatively high As content. In the Chiu-Chiu village without As removal plant, in 1998, drinking water contained 750 - 800 µg L-1 of arsenic at home [5]. At present, the World Health Organization recommend a guidance value of 10 µg L-1.
The chronic impact of As in the Region produces cancer and non-cancer health outcomes [6]. Cancer effects [7] are in a similar way than in other well documented environmental As exposures of Taiwan [8], India, Bangladesh, USA [9], Mexico [10], Argentina and Hungary [11]; different type of cancer, mainly lung and bladder cancer have been reported as main problems arsenic associated diseases. According Smith., et al. [12], the impact of arsenic on the population mortality in the II Region of Chile is greater than that reported anywhere to date from environmental exposure to carcinogen in a major population; dose - response relation between arsenic concentration in well water and mortality from cancers has been characterized [13].
Typical clinical non cancer effects affecting the healthy life quality of the persons, associated with the inorganic arsenic chronic ingestion through drinking water or environmental exposition have been studied and is reported in numerous papers. Vascular diseases, abnormal pigmentation, Raynand´s syndrome, acrocyanosis, hyperkeratosis, gangrene of fingers, ischemia of the tongue, diabetes, thrombosis, cerebral vascular disease, especially cerebral infarction, coronary artery occlusions and other cardiovascular diseases (CVD) has been associated with arsenic exposure [14]. Less common complications such as liver enlargement (hepatomegaly), spleen enlargement (splenomegaly) and fluid in the abdomen also has been cited [15].
Smoking, high serum low density lipoprotein cholesterol and high blood pressure have been shown to promote atherosclerotic disease in man. Yet these factors explain only about 60 per cent of the incidence of coronary heart disease (CHD) or CVD. Heart failure is not a uniform disease entity, but a syndrome with various causes, including hypertension, ischemia and congenital heart disease, cardiomyopathy, myocarditis and intoxication [16]. As far as CVD are concerned, there is epidemiological evidence that trace elements may play a role [17]. However, the overall outcome of the cardiovascular disease epidemiological research has been to confirm the existence of water-related factors, independent of socio-economic or geographical influences on CVD. It has not been possible to identify a specific water constituent as responsible, and there is little hope of doing so by epidemiological research. Also, the nutritional evidence indicates that Cu is an antioxidant nutrient linked to an impressive array of biological mechanisms associated with CVD, however, most data arise from animal studies where induced copper deficiency was severe [18]. Nevertheless, even more direct evidence respects the trace elements concern is necessary. Accordingly, recently it has been demonstrated that the effect of Ni on human cardiac tissue was related to oxidative stress [19]. Appear not be apparent that the more direct evidence such as the speciated arsenic status in cardiovascular related tissues should be central to any discussion of trace elements and CVD.
The mechanism across mammalian animals including man metabolises and detoxify inorganic As is methylation to methylarsonate and dimethylarsinate [20]. Before this methylation arsenate must be reduced to arsenite; the binding of arsenite to tissue proteins would be an additional or perhaps the first step in the detoxification of inorganic As prior to methylation [21]. Therefore, before methylation, increases the in vivo toxicity and risk to the organism [22]. Otherwise, recently has been informed that biomethylation being a process that potentiates toxicity and carcinogenicity of inorganic arsenic [23].
In autopsy samples of people exposed to environmental basal arsenic levels, the concentration of the element is very similar in all internal organs (0.1 µg g-1) but slightly higher in hair (0.6 µg g-1) and nails (0.4 µg g-1). However, after an acute lethal dose (8.0 g of As2O3), the highest levels were observed decreasing order in liver, kidney and other organs (muscle, lungs, brain). The inorganic trivalent species was predominant (> 80%) and the metabolites MMA and DMA represented 10 and 5% respectively except in lipid rich organs that concentrations of these species are higher [24]. In animals arsenate has been shown to incorporate in the skeleton due to its similarity with phosphate anions. Background levels in breast milk range between 0.1 and 1 ng L-1, but concentrations multiplied by a factor of 4 have been observed in human in case of regular seafood consumption [25] and by a factor of 8 in case of consumption of drinking water containing 200 µg As L-1 [26]. Other data indicates that arsenic can easily crosses the animal and human placentas [27].
The present study deals with the determination of total As content and main inorganic and organoarsenic species found in three heart tissues (auricle, mammary artery and fat) and the saphene vein (used as by-pass) of people chronically exposed to arsenic in the Chilean II Region, suffering cardiovascular diseases (CVD) and subjected to hearth surgery in Antofagasta. The found concentrations of total As and As species, and the histology study of some of these tissues, contribute to a best understanding about if As or its main species could be involved in the heart disease.
Auricle tissue, saphene vein, mammary artery and heart fat tissues from about 200 patients operated in the Antofagasta Clinic during 2015 and 2018 of coronary thrombosis were analysed for total As content. The samples were obtained from a population under study, made up of patients who have lived at least five years in the II Region of Chile, and population control samples were obtained from patients operated in the Catholic University Clinic in Santiago of Chile, which coming from Chilean Regions without arsenic problem.
All manipulations and procedures for the preparation of the samples were made in the bench of the “clean laboratory” inside a laminar flow hood (Labconco, Purifer Class II) using inert devices such as plastic and Titanium knives, agate grinding mortar, and scalpels, scissors and forceps of surgical stainless steel. In each case, then of be liberated of the Titanium clasp and the fats residues, the tissues were rinsed with deionised water and separated as single samples, except the fat of which was prepared as pooled sample. All samples were stored at -20ºC before uses. Dry weight/ wet weight factors were obtained according UNEP protocol [28] at 60ºC.
From these samples, three different groups have been chosen for the study, results presentation and discussion: Group I. More contaminated group formed by 20 people presenting the higher Arsenic content in the auricle (the tissue with higher mean As level). Group II. Comparison group formed by 15 people presenting the lower As content in the auricle (probably the heart problem is not due to As); and Group III. Control group formed by 10 people suffering heart attack, which coming from V, VIII and IX Chilean Regions which are not exposed to the impact of arsenic (Valparaiso, Concepción and Temúco). Group IV. An additional samples from similar heart tissues were obtained for basal level from died persons living in Madrid where As contamination is not expected. Before operation, all the Chilean people initially analysed, filled in a survey with some personal characteristics for later relation with the As content (Table 1).
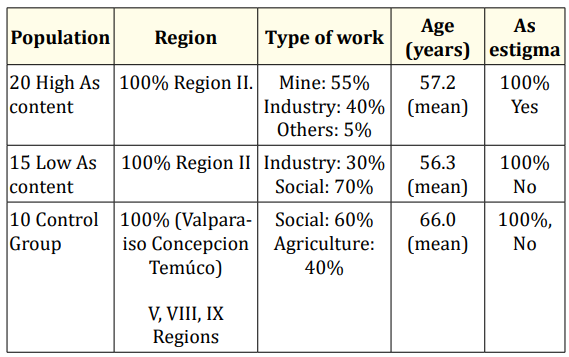
Table 1: Global characteristic of people under study.
For the histological study performed in some samples, the tissue was washed for 24 hours in running water and fixed in paraffin blocks dyed with hematoxilin-eosine, trichromic of Masson and orceine Van Giesson [29]. The histochemical methods it’s based on combination of chromogenic reagents with mixtures of masking agents that block the reactivity of tissues. The paraffinzed sections show green granules, insoluble in water, through dissolved by acid and by ammonium hydroxide. This procedure demonstrates pretty well every practical histopathologic with additional diagnostic studies such as histological examination in microscopic scission.
The interval and mean value for total As found in the samples of the four analysed groups are given in table 2. In the Group I (more contaminated), the auricle has by far the higher concentration, with a mean value of 7.7 µg g-1, dry tissue, following by the saphene vein with 2.5 µg g-1. Mammalian artery and fat tissues in these groups present similar but lower As concentration than the former tissues with about 1 µg g-1 each. The total As concentrations found in the auricle (and also saphene vein) of some people, not chosen as components of the Group I, are really high of about 20 µg g-1 that reveal the high impact of the As in some individuals living and working in the contaminated areas. The comparison between Group II (less As contaminated in the Region) and Group III (people from V, VIII an IX Chilean Regions), shows that concentration in the different tissues are quite similar. Considering the long distance between both population groups, these values can be considered as the mean level in these tissues for Chilean people.
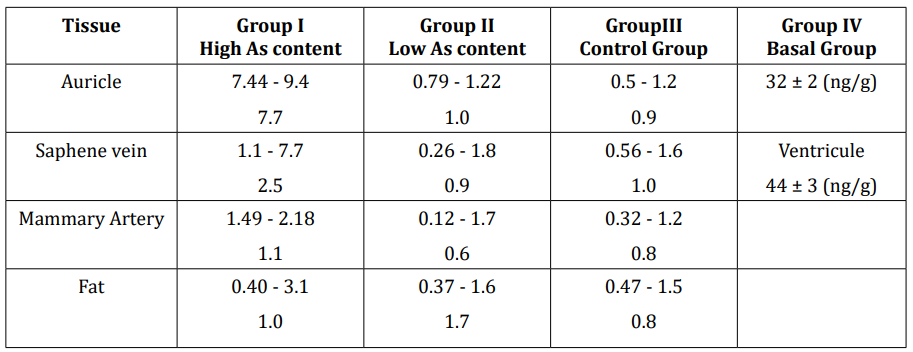
Table 2: Concentration intervals and mean value for total As in the analysed tissues for the different analysed groups (µg g-1).
LUTS-1: Found: 2.7 ± 0.2 certified: 2.83 ± 0.13. DORM-1: Found:17.0 ± 0.8 certified: 17.7 ±1.2.
However, they are quite high comparing the basal level of the whole heart tissue of about 0.003 - 0.026 µg g-1 (48) dry tissue and the Group IV of a person living in Madrid.
Figure 1 shows the ratio between mean values for the auricle and the other tissues in the same population group. Figure 2 shows the ratio for the same tissue between the three Chilean groups analysed. As we can see, Group I support the higher difference between the auricle (and in less extend the saphene vein) and the other tissues.

Figure 1: Total As relation between auricle tissue and saphene vein, mammary artery and fat tissue in each of the considered Groups.
Figure 3 and 4 shows the histological plates for the saphene vein of a damaged and non-damaged tissue respectively. The vascular pathology, figure 3 is type concentric intimal fibrosis. This vein-damage consists in the expansion of the internal surface due to myofibroblasts and due to this expands; the light circumference is strongly reduced. In this case, the tissue is seriously ill and the intimal layer has analogous thickness than the muscular layer. The concentric intimal fibrosis does not belong to the principal atherosclerotic disease damage, main causes for heart attack and is produced by age, or by the influence of chemical or immunological processes. In our case, the person is relatively young (45 years old) and can be suspected of arsenic as the main cause of the tissue damage. Then, appear not to be apparent that arsenic may cause necrosis in cardiovascular tissues of the man [30]. However, at the light of the new knowledge, a critical issue is how to distinguish between apoptosis and necrosis [31]. The total As concentration found in the saphene of this patient was of 2.1 µg g-1.

Figure 2: Total As relation between the same tissue (auricle, saphene vein, mammary artery and fat tissue) in each of the considered Groups I and II.

Figure 3: Histological study of a saphene vein suffering concentric intimal fibrosis.

Figure 4: Histological study of a a normal saphene vein.
Six species were considered for As speciation in the tissues. Arsenite and arsenate they are the main contaminant species. MMA and DMA both are the main products of the cellular biomethylation through the conjugated effects of S-adenosylmethionine and the methyltransferase enzyme. AsB and AsC they can enter the human organism through food, mainly of marine origin. The detection limits for total As was 5.0 ng g-1 and for As species were 3 - 5 ng g-1 for As(III), 6 - 8 ng g-1 for As(V), 3 - 5 ng g-1 for AsB, 4 - 5 ng g-1 for MMA and 6 - 7 ng g-1 for DMA, in the different samples tested.
The four tissues (auricle, saphene vein, mammary artery and fat) of some people from the Group I has been analysed for As species.
Table 3 contains the total As concentration, extraction efficiency on methanol-water 1:1 (two consecutive extractions), and methanol water 9:1 (after the two first extractions) in the above cellular residue and % of non-extracted As retained in the cellular residual material.
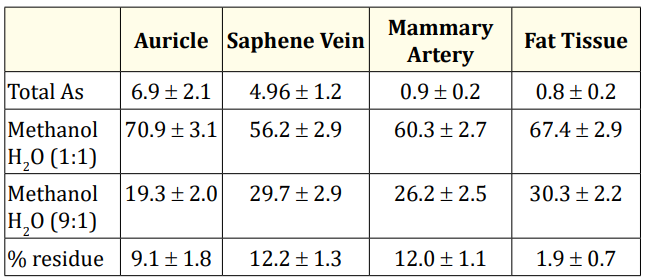
Table 3: Fractionation of total Arsenic concentration (µg g-1 dry weight) in cardiovascular tissues expressed in extraction efficiency (%) on methanol-water 1:1, and methanol water 9:1 (see text) and % of non-extracted As on the residue.
Figure 5a-5d shows the percentage of each of the present species of this person. Similar chromatograms and species were obtained for other people in this groups.

Figure 5: Percentage of As extractions and As species referred to dry tissue for: (a) auricle, (b) saphene vein (c) fat and (d) mammary artery tissues after methanol-water 1:1 extraction.
First of all it is important to point out that most of the As present in all the tissues is extracted in the methanol-water 1:1 extract (Table 3). Methanol-water 9:1 extract coming from the cellular residues resulted be the preferred medium for organoarsenic compounds, but more works are in progress to this respect.
The figure 5a can be seen that in the case of auricle, only As(III) and As(V) are the species present in the tissue. After the first methanolic extraction which contain to the cytosol solution, the As(III) extracted was about 50% of the total As content in the tissue. The As(V) is about 10% of the total. This result is interesting because As(V) is the main contaminant species present in the natural waters, therefore biotransformation to As(III) is a main mechanism. No other species seems to be present. Both inorganic As species were confirmed by spiking with standard solutions of the species. Completely symmetrical increases of the peaks were obtained. As(III) was also characterised using the cationic column Hamilton PRP X200 in which As(III) is not overlapped with other possible species.
In the saphene vein also As(III) and As(V) are the main species present, but little amount of other species such as DMA was found. One again the As(III) is the predominant species in the methanolic extract, which could involve that free As(III) specie is a principal component of the cytosol solution in this cardiovascular tissues.
In fat tissue AsB is the predominant species and in the mammary artery the As(V) species, that account about 40% and 30% respectively of the total As content in the respective tissues.
Dorm-1 and Tort-1 species have also been quantified and although only total As is certified in both materials, our species concentration are close to the reported in others works [32]. Our results were in µg g-1, for Dorm-1: AsB (15.0) and DMA (0.54) and the reported paper from others are AsB (15.5 - 14.1) and DMA (0.60). For the Tort-1 material, we found AsB (15.3), DMA (1.41) and As(V) (0.30) and AsC (n.d.), and the reported results were: AsB (16.0), DMA (1.64, and 1.01), AsC (0.04), and As(V) (0.39) [33].
It is known that inorganic arsenic species have a high affinity for proteins containing conjugated sulphur groups and therefore extracellular and intracellular proteins could bind As. Although inorganic As(III) is the main species bounded to proteins, some other studies report, for example, that in the rat liver intracellular solution, about one half of the methylated As species were protein bound [34]. Treatments such as precipitation of proteins with 5% trichloroacetic acid, thiol - reactive agents (ex. N-ethylmaleimide, β-mercaptoethanol) or chelators (ex. 2,3 dimercaptopropanol) failed to release most protein-bound to arsenicals. Treatment with CuCl2 releases about 90% of all protein-bound arsenicals [35]. The fact that the methanol - water 1:1 and 9:1 extracting solutions yield higher than 80% of As species in most of the biological samples and also in the heart tissues analysed in this work, suggest that intracellular As species can be extracted in these extracting mixtures.
Transporters as carrier proteins, channels and ion pumps have all been regarded as mechanisms that are capable of permitting the movement of ions across the cell membranes by both electrochemical gradient and the different composition of intracellular and extra cellular fluids [36].
One surprising fact is that the DMA and MMA species are absent in the auricle tissue and the concentration of DMA is very low in the saphene vein in Group I, mostly considering that these species are the main species formed in the detoxification mechanism. Some explanation can be highlighted: i) the lack of methylation mechanisms because of the lack of methylating agents in these tissues; ii) at a certain inorganic As dose level, the methylation efficiency decreases in animals and humans [37]; iii) the incapability of DMA to bind some cell constituents as in the case of lungs of rabbits has been found [38].
The presence of high total As and high As(III) species content in the auricle and saphene vein of more contaminated people (Group I), the damage found in the saphene vein tissue and the global characteristics of the people under study in which the As stigmas are present in all of them, suggests that As could be involved in the cardiovascular diseases (CVD).
This research was supported by a grant under the EU´s RTD programme and the BQU 2002-01348 projects.
Copyright: © 2020 Isabel Pizarro Veas., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.