Mir Mohammad Yusuf*
Assistant Professor, Critical Care Pediatric, Bangladesh Institute of Child Health (BICH), Dhaka Shishu (Children) Hospital, Bangladesh
*Corresponding Author: Mir Mohammad Yusuf, Assistant Professor, Critical Care Pediatric, Bangladesh Institute of Child Health (BICH), Dhaka Shishu (Children) Hospital, Bangladesh.
Received: February 29, 2020; Published: April 29, 2020
Citation: Mir Mohammad Yusuf. “Acid-Base Disturbances: A Key Concept to Prevent Life-Threatening State of Sick Children”. Acta Scientific Paediatrics 3.5 (2020):13-23.
Essentially all sick children, can lead to acid-base disturbances. Therefore, acid-base disorders need to be anticipated in all critically ill pediatric patients. Monitoring of the acid-base status will allow the early recognition of derangements and the prevention of what could become a life-threatening state. Acidosis is the most common acid-base derangement in the pediatric intensive care unit (PICU), with metabolic acidosis pH of < 7.2 potentially indicating a more severe course and worse outcome. Further assessment of the type of acidosis and the presence of a mixed acid-base disorder requires measurement of pCO2, serum bicarbonate and calculation of the anion gap. The most commonly encountered causes of metabolic acidosis in the PICU are sepsis, renal insufficiency and DKA, while Respiratory distress syndrome (RDS), Meconium aspiration syndrome (MAS) and Severe Status Asthmaticus are the usual suspects in respiratory acidosis. Alkalosis, on the other hand, is less common in the PICU. Fluid status derangements and, especially, gastric fluid depletion are the usual underlying causes of metabolic alkalosis, whereas rapid respiration secondary to lung diseases, excessive mechanical ventilation, or central nervous system diseases are the common causes of respiratory alkalosis. In the PICU, identification of acid-base derangements is followed by timely stabilization of the patient irrespective of the underlying cause. Depending on the severity of the derangement and the patient’s response to the stabilizing interventions, the underlying cause might also need to be aggressively sought and emergently reversed. Identification of the underlying cause(s) of the acid-base disorder at hand may be the final step in the management of these patients, but plays an important role both in the prevention of worsening of the derangement and other complications as well as in the determination of the patient’s overall prognosis.
Keywords: Acid-Base Disturbances; Sick Children; Life-Threatening State
Acid-base balance is one of the body’s most important homeostatic mechanism. It represents equilibrium, balance, and a steady state. The human organs and tissues function under a tightly controlled pH in the range of 7.35 to 7.45. Depending on the degree of deviation of pH outside this narrow range, several homeostatic responses are activated in an effort to maintain a stable extracellular pH for optimal cellular function and thus to restore normal acidbase status [1]. Therefore, Acid-base disorders reflect the seriousness of the underlying disease and are responsible for morbidity and mortality in sick children [2]. Understanding of acid-base dysfunction in various pathological conditions of critically ill children is an asset to a pediatrician in efficient treatment about patient assessment, therapeutic decision and prognosis of the patient [3].
Disorders of acid-base balance can create complications in many disease states, and occasionally the abnormality maybe so severe so as to become a life-threatening risk factor. Several factors impact the prognosis of patients with acid base disturbances like severity of acidemia, acuity and duration of the derangement, functional status of the major organs especially lungs and kidneys and last but not the least the underlying cause [1]. Initially reactions by chemical buffers will attempt to neutralize the derangement, followed by ventilator adjustments by the lungs and finally alterations in acid excretion by the kidneys [1].
Hence a thorough understanding of hemoglobin-oxygen interactions and gas exchange provide cornerstone for clinical success to Pediatric care. Critically ill children commonly have acid-base disorder [4]. Blood gas measurements help in the diagnosis of metabolic and respiratory acidosis associated with birth process and with postnatal adaptation to air breathing [4-6]. The cardiovascular system undergoes changes after birth, respiratory gas exchange begins instead of formerly placental function, must be established by the lungs within minutes. Therefore, frequent and serious difficulties in cardio-respiratory adaptation in perinatal and neonatal periods are not surprising [7].
Blood gas analysis provides pH, PCO2 from which HCO3- and base excess (BE) can be derived [8-11]. Moreover, it is easily understandable and widely used at the bed side management [12]. This traditional approaches to analysis of acid-base status adapted from Henderson-Hasselbalch equation mathematically links the variables of pH, pCO2 and bicarbonate concentration (HCO3) [13]. The pCO2 concentration in a given patient reflects the balance between metabolic production of CO2 and excretion by ventilation. The normal range of pCO2 after the first hours of life can be considered 35 - 45 mmHg, desirable CO2 values for a specific situation may be either higher or lower [14,15]. In this regard marked structural and functional difference found in children in comparison to adults i.e. children have narrow distal airways, therefore atelectasis develop quickly resulting in rapid-onset of hypercarbia and hypoxia. Chest wall is compliant and respiration is less efficient; the respiratory center is immature, hypoxia and hypercarbia lead to decreased respiratory drive. In addition, they have reactive vascular bed to maintain their blood pressure until late, therefore one cannot rely on hypotension to diagnose shock as in adults [16]. Hence blood gases provide essential information on acid-base status in critically ill neonates and predict their mortality. Perinatal asphyxia and neonatal sepsis both are common occurrence in neonate and major health problems in developing countries and devastating cause of mortality. The acid-base abnormalities are common in perinatal asphyxia and neonatal sepsis, which need more vigorous measures to reduce their mortality in an emergency situation.
Sometimes, perinatal asphyxia occurs when there is inadequate placental gas exchange to meet ongoing tissue needs for oxygen consumption and CO2 elimination. The combination of lactic acidosis, product of anaerobic metabolism and CO2 accumulation results in a mixed acidosis. It results most commonly from a drop in maternal blood pressure or some other substantial interference with blood flow to the infant’s brain during delivery. This can occur due to inadequate circulation or perfusion, impaired respiratory effort, or inadequate ventilation [17]. An infant suffering from severe perinatal asphyxia usually has cyanosis, less perfusion, poor responsiveness, reduce muscle tone and poor respiratory effort as reflected in low APGAR score (5-minute). Extreme degrees of asphyxia can cause cardiac arrest and death. There has been a scientific debate whether newborn infants with asphyxia should be resuscitated with 100% oxygen or normal air [18]. It has been demonstrated that high concentrations of oxygen lead to generation of oxygen free radicals, which have a role in reperfusion injury after asphyxia [19]. Immediately after birth asphyxia, hypothermia generally lower metabolic rates and diminishes the glutamate levels in brain. In neonatal sepsis, unstable temperature and less tissue perfusion leading to derangement of acid-base balance. As temperature affects pH, pCO2 and pO2 [14]. Hence, it is desirable to have values corrected for patient temperature.
Hydrogen ion (H+) is much more precisely regulated in the extracellular fluid in order to achieve a concentration of 0.00004 mEq/L compared with sodium, for example, which is maintained at 135 - 145 mEq/L. This precision with which H+ is regulated emphasizes this ion’s critical impact on cellular functions. Protein is the largest source of hydrogen ions (65% of total). The remainder from the incomplete catabolism of carbohydrate, fats and organic acids as pyruvic, lactic, acetoacetic and citric acids. This hydrogen ion is buffering by ICF, ECF followed by respiratory and finally the kidneys excrete the hydrogen ions to maintain balance. By definition, an acid is a substance that has at least one H+ and can donate H+ ions when in a solution, and a base is a substance that can accept H+ ions [20]. A strong acid rapidly dissociates and releases large amounts of H+, such as hydrochloric acid, whereas a weak acid, such as carbonic acid, releases H+ with less vigor. Similarly, hydroxides are strong bases, while bicarbonate (HCO3−), phosphate, and proteins are weak bases. Most acids and bases in the extracellular space are weak, but they constitute the body’s principal buffers. Further, due to higher metabolism, production of acid is three times more than adult. Respiratory and renal function cannot maintain acid-base balance properly as adult.
The two classes of physiologically produced acids are volatile acids, also known as carbonic acid (H2CO3), and fixed acids, also known as non-carbonic acids. The metabolism of carbohydrates and fats generates approximately 10,000 - 15,000 mEq of CO2 daily which in turn results in increased carbonic acid. The lung is the main organ charged with the elimination of volatile acids. The metabolism of proteins, on the other hand, generates fixed acids. Approximately 100 meq of fixed acids are generated daily from ingestion and metabolism [21]. The kidneys are the only organs capable of eliminating fixed acids through excretion in the urine. The resulting extracellular level of H+ is approximately 40 nEq/L (30 - 60 nEq/L). As a result of this disproportionate degree of production of volatile acids compared with fixed acids, the lung plays a profound role in acid-base status. Acute respiratory failure and inability to eliminate CO2 as a result of airway obstruction would result in a significant rise of pCO2 and corresponding drop in pH that would overwhelm the cellular buffers and the kidneys’ acute compensatory capabilities. On the other hand, acute renal failure and consequent inability to eliminate fixed acids, in the absence of pathological sources of non-carbonic acids, would result in a much milder and less acute derangement.
The pH of a solution is the negative logarithm of H+ concentration as defined by pH = −log [H+]. As CO2 dissolves in a solution, it dissociates into carbonic acid following the Henderson-Hasselbalch equation: H2CO3óH++ HCO3-. The dissociation constant of carbonic acid follows the law of mass action and is as follows: Ka = [H+] × [HCO3−]/[H2CO3]. Given that the concentration of H2CO3 is proportional to that of dissolved CO2Ka = [H+] × [HCO3−]/[CO2]. After logarithmic transformation into logKa = log[H+] +log[HCO3−]/ [CO2] and rearrangement into −log[H+] = −logKa + log[HCO3−]/ [CO2], it follows that pH = pKa + log[base]/[acid], given that pH is the negative logarithm of H+ concentration. Normal acid-base status is maintained by the pulmonary excretion of carbonic acids and by the renal excretion of non-carbonic, fixed acids, and formation of bicarbonate. Hence, the last equation could be envisioned to be as follows: pH is proportionate to kidney [HCO3−] over pulmonary [pCO2]. Therefore, pH increases with increasing HCO3−, the numerator, and declines with increasing levels of pCO2, the denominator.
pH = pK + log (HCO3/H2CO3) [pK is constant, it is pH value at which H2CO3 is dissociated i.e. concentration of HCO3- and carbonic acid in body are equal. pK = 6.1 for H2CO3]. Normal ratio HCO3-/ H2CO3 = 20/1 and hence pH = 6.1 + log20 = 6.1 + 1.3 = 7.4. [pH Normal = 7.35 - 7.45. Alkalosis > 7.5, Acidosis < 7.3. Severe Acidosis < 7.2].
Once derangement occurs, H+ concentration is corrected in a timely and stepwise approach starting with chemical buffers, followed by pulmonary ventilation and finally renal control of acidbase excretion.
Chemical buffers are available in both extracellular and intracellular compartments. They respond within minutes to neutralize derangements. Chemical buffers are naturally occurring weak acids and bases. They impart their correction on systemic pH by converting strong acids or bases into weak acids or bases, thus minimizing alterations in pH. There are three buffering systems that are recognized:
The bicarbonate buffer system is the most powerful of all the three systems in the extracellular space, while proteins dominate the intracellular buffering compartment. Depending on the severity of the derangement and its chronicity, the limited amount of chemical buffers may not be capable of completely ameliorating the derangements and correcting the pH.
The rate of response by the respiratory and renal mechanism differs. Respiratory responses occur more rapidly: 50% in 6 hour and 1005 in 14 - 60 hour. Renal mechanism are slower with renal base excretion more rapid than acid excretion. Renal base excretion is 50% at 80 hr and 100% at 24hr. Renal acid is excretion is 505 at an approximately 36hr and is 100% at 72hr.
The lungs respond to deviations in pH by altering the rate and depth of ventilation. The lungs can only eliminate or retain CO2. Peripheral chemoreceptors in the carotid and aortic bodies respond within minutes to changes in pO2, pCO2 and pH. On the other hand, central chemoreceptors in the cerebral medulla are sensitive only to pCO2 with a slower but stronger and more predominant response [22]. Arterial pCO2, therefore, is the most important factor in altering ventilation. These pulmonary responses typically begin in the first hour and are fully established by 24h [23]. Pulmonary regulation, however, is only 50 - 75% effective in restoring H+ concentration all the way back to normal when the primary process is metabolic, as the lung is only capable of eliminating CO2 and not fixed acids. Nevertheless, the pulmonary buffering system is at least as effective as the chemical buffering system.
The kidneys correct extracellular pH by controlling serum bicarbonate concentration through the regulation of H+ excretion, bicarbonate reabsorption, and the production of new bicarbonate. The kidneys excrete H+ in combination with phosphate (HPO42- + H+→ H2PO4-), other acids, or with ammonia to form ammonium [24]. When blood acidity is significantly increased, glutamine is proportionately metabolized into ammonia. Ammonia, in turn, serves as the recipient of H+. Whereas the lungs can eliminate or retain only volatile acid, namely pCO2, the kidneys can eliminate or retain both acids and bases and are the primary removal site for fixed acids. Renal compensation is the last process to join other buffering forces but insures complete correction over time. Renal compensation typically begins in the first day and is fully established in 3 - 5 days.
In the ICU, it is not infrequent to encounter patients with two or more acid-base disorders. This type of complex presentation is easily recognized whenever the measured compensatory values of either bicarbonate or pCO2 differ significantly from what would be expected [25,26]. For example, in a patient with primary metabolic acidosis, a bicarbonate level of 14 mEq/L should be adequately compensated by hyperventilation that decreases pCO2 to 28 mmHg (for every 1 mEq decline of bicarbonate, pCO2 declines by 1.3 mmHg in compensation).
In neonates, ABG is routinely indicated in patients with shock or respiratory distress who may either need ventilation and to monitor progress of patient on ventilator to act correctly. The acid-base status in major pathological disorders occurring in neonates such as birth asphyxia, bronchopneumonia, sepsis, HDN, amniotic fluid gastritis etc. Birth Asphyxia and Sepsis constituted major cause of admission among sick neonates and metabolic acidosis is the predominant acid-base abnormality which is established [27-30]. pO2 values vary considerably throughout the day in sick neonates and may be lower in premature baby due to reduced lung function [12].
ABG measurement may also give important prognostic information and early warning signals. Therefore, acid-base disorders need to be anticipated in all the critically ill neonates. Regular monitoring of the acid-base status will help in early recognition of the underlying cause and also help in prevention of life-threatening state in sick neonates in relation to various pathological conditions especially with common conditions like birth asphyxia, sepsis and septic shock.
(1) Severe respiratory or metabolic disorders (2) Clinical features of hypoxia or hypercarbia (3) Shock (4) Sepsis (5) Decreased cardiac output (6) Renal failure (7) Ideally any baby on oxygen therapy (8) Inborn errors of metabolism.
Goals of ABG is to characterize the type of disorder, quantify the magnitude and asses the nature and extent of compensation.
Ideal artery for sampling in newborn is radial or umbilical artery. Venous blood is good for HCO3- estimation but bad for pH, pCO2 and pO2 (pH and pO2 level are lower but pCO2 is higher than Arterial blood).
Normal values and range of ABG parameters pH: 7.40 (7.35 - 7.45). pCO2: 40 (35 - 45) measures H2CO3. HCO3-: 24 (22 - 26), pO2: 100 (90 - 100),
Base Excess: +2.5 to -2.5 (In neonate may be 10).
Just the numerical value doesn’t tell normalcy, all values are to interpreted in the context of each other and with clinical condition.
Remember by heart: (CO2 is a respiratory acid).
pH and HCO3-: Moves in same direction. pH and pCO2: Moves in opposite direction.
HCO3- and pCO2: Moves in same direction (simple disorder).
HCO3- and pCO2: Moves in opposite directions (Mixed disorder). pH remains normal in all compensated states.
pO2 is variable according to gestational age e.g. < 28 weeks 45 - 65 whereas 28 - 40 weeks 50 - 75 mm of Hg. Even pO2 varies term neonatal age e.g. pre-birth (Scalp) 25 - 40, 5 minutes after birth 49 - 73, 1 - 7 days after birth 70 - 75. In Children pO2 = 70 - 100 mm of Hg [14]. Hemoglobin is required to calculate oxygen content of blood. Patient with anemia may have normal saturation because of cardiac compensation but decreased oxygen content as less hemoglobin is available for transporting oxygen. Hence O2 supplement much variable in aspect of general condition and different entity [12]. Pulse oximetry measures peripheral O2 saturation (SaO2) not pO2 and this is insensitive to detecting hyperoxaemia [14].
Bicarbonate is a byproduct of body’s metabolism. Blood brings bicarbonate to lungs, and then it is exhaled as carbon dioxide. Kidneys also help regulate bicarbonate. Bicarbonate is excreted and reabsorbed by kidneys. This regulates body’s pH, or acid balance [15].
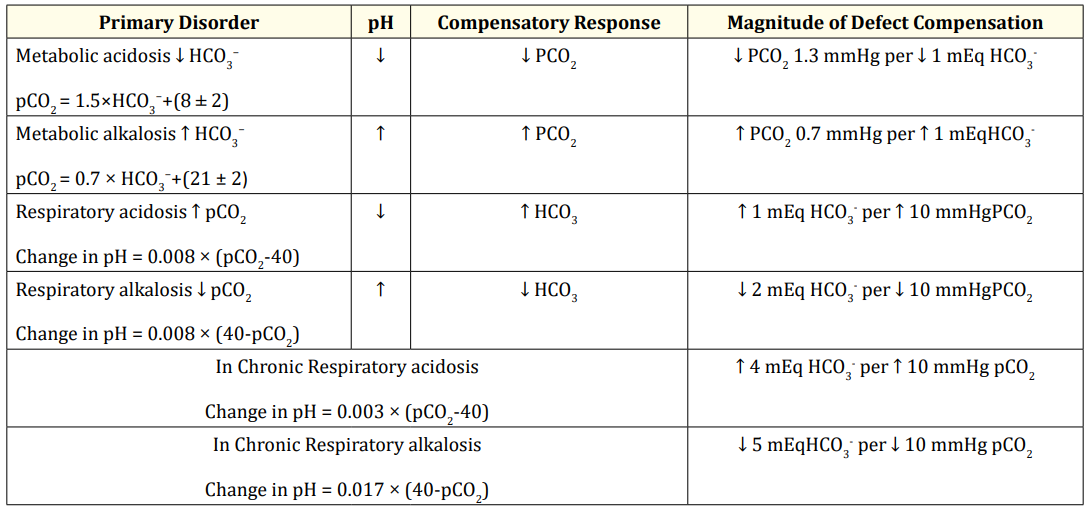
For every acid-base deviation, there is an appropriate compensatory response that follows a very predictable pattern. As was shown earlier, pH is determined by the ratio between the HCO3 concentration and pCO2 and not by either value in isolation. As such, processes that result in deviation in serum bicarbonate are compensated for by the lungs, which control pCO2 and processes that result in deviation in pCO2 are corrected by the kidneys, which regulate bicarbonate. In metabolic acidosis, for example, a low HCO3−/pCO2 ratio causes a decline in pH, resulting in stimulation of peripheral chemoreceptors, which, in turn, increase ventilation to decrease pCO2. Given that CO2 is an acid, its fall causes the pH to increases back toward normal. In metabolic alkalosis, on the other hand, a high pH induces hypoventilation through peripheral chemoreceptors, resulting in a rise in pCO2, which, in turn, lowers the pH. This latter response is limited by the degree of the resulting hypoxemia induced by hypoventilation, rendering pulmonary compensation for an increased pH not nearly as effective as for a reduced pH. A very convenient approach to recall the appropriate compensatory mechanisms to the primary disorders is that bicarbonate and CO2 vary in the same direction (e.g. a fall in bicarbonate is compensated for by a fall in pCO2 and vice versa), as one is an acid and the other is an alkali, each with biologically equivalent potential to neutralize the primary derangement [1].
Simplified approach to analyze Acid-Base status: In order to understand the various processes that can co-exist in a patient, one must systematically evaluate the blood gases and serum electrolytes. The article uses 6 simple steps to analyze the acid-base status of the patient.
If pH is altered it is uncompensated disorder. When pH is normal it is difficult to distinguish primary change from compensatory change.
Blood gas analyzer (Gastat-600) based on the principle of potentiometry analyzed pH, PCO2, by respective electrodes. Base excess (BE) and [HCO3] were calculated parameters from pH and PCO2 were provided by the analyzer. Electrolyte analyzer (Rapid lab 1265) based on the principle of potentiometry analyzed Na+, K+, CI-. Anion gap (normal reference values ranged from 10 to 20 mEq/L plasma) was calculated from the following formula. AG = [Na+ + K+]- [CI-+ HCO3-] [31].
If the gap is greater than normal, then high anion gap metabolic acidosis is diagnosed e.g. lactic acidosis, diabetic ketoacidosis. In patients with a normal anion gap the drop in HCO3- is the primary pathology. Since there is only one other major buffering anion, it must be compensated for almost completely by an increase in Cl-. This is therefore known as hyperchloremic acidosis. The HCO3- lost is replaced by a chloride anion, and thus there is a normal anion gap e.g. Gastrointestinal loss of HCO3- (i.e. diarrhea) (note: vomiting causes hyperchloremic alkalosis), Renal loss of HCO3- (i.e. proximal renal tubular acidosis also known as type 2 RTA), Renal dysfunction (i.e. distal renal tubular acidosis also known as type I RTA), Renal Hypoaldosterone (i.e. renal tubular acidosis also known as type IV RTA) characterized by elevated serum potassium. Ingestions (NH4Cl and acetazolamide), Some cases of ketoacidosis, particularly during rehydration with Na+ containing IV solutions, Mineralocorticoid deficiency (Addison’s disease). Note: a useful mnemonic to remember this is FUSEDCARS (fistula-pancreatic), urtero-enterostomy, saline administration, endocrine (hypoparathyroidism), diarrhea, carbonic anhydrase inhibitors (acetazolamide), ammonium chloride, renal tubular acidosis, spironolactone). Low anion gap is frequently caused by hypoalbuminemia. Albumin is a negatively charged protein and its loss from the serum results in the retention of other negatively charged ions such as chloride and bicarbonate. As chloride and bicarbonate anions are used to calculate the anion gap, there is a subsequent decrease in the gap [31].
Expected compensatory responses to acid-base disturbances (Primary Acid-Base disorders)
In metabolic acidosis, the expected pulmonary compensation is a 1.3 mmHg fall in pCO2 for every 1mmol/L reduction in bicarbonate concentration [32]. In metabolic alkalosis, on the other hand, the pulmonary compensation raises pCO2 by 7 mmHg for every 10 mmol/L elevation in bicarbonate concentration [33,34].
In respiratory disorders, the compensatory mechanisms are biphasic: The first phase is acute and dominated by chemical buffering mechanisms, while the second, chronic phase is dominated by renal responses. In acute respiratory acidosis, the serum bicarbonate concentration rises 1 mmol/L for every 10 mmHg increase in pCO2, whereas this ratio increases to 4 meq/L per 10 mmHg in chronic respiratory acidosis. This latter renal compensation is the result of neutralization of H+, initially by phosphate and subsequently by ammonium excretion [35,36]. It is essential to recognize that the renal response is tightly regulated, in that the provision of medical bicarbonate results in the urinary excretion of the excess alkali with no change in the plasma HCO3 or pH [35].
In acute respiratory alkalosis, bicarbonate concentration falls by 2 mmol/L for every 10 mmHg decrease in the pCO2, whereas this ratio becomes 5 mmol/L-1 per 10 mmHg in chronic respiratory alkalosis [37,38]. This serum bicarbonate decline is achieved by decreased urinary bicarbonate reabsorption and ammonium excretion [39].
Table
Utilization of an arterial blood gas (ABG) analysis becomes necessary in view of the following advantages: Aids in establishing diagnosis, Guides treatment plan, Aids in ventilator management, Improvement in acid/base management which in turn allows for optimal function of medications, Acid-base status may alter electrolyte levels that may be critical to a patient’s status [40]. The term ABG refers to a specific set of tests performed on arterial blood sample. It provides four key aspects of information: pH, pO2, HCO3 and pCO2. ABG gives valuable information regarding patient’s oxygenation and acid-base status.
If pH < 7.25 stimulation of respiratory centre occurs but if < 7.0 depression will occur. Recovery is unlikely to occur if the blood pH falls below 6.8 or increases above 7.80.
The pCO2 concentration in a given patient reflects the balance between metabolic production of CO2 and excretion by ventilation. pCO2 elevation of 10 mmHg decreases pH by 0.08 while pCO2 decrease of 10 mm Hg, increase pH by 0.08 [14]. Hypercapnia pCO2 > 50 mmHg, Hypocapnia pCO2 < 30 mmHg. Type I Respiratory Failure involves ↓pO2 and normal or ↓pCO2 [require oxygen therapy e.g. humidified high-flow oxygen therapy, CPAP (continuous positive airway pressure) to achieve adequate oxygen saturations] [40] whereas Type II Respiratory Failure involves ↓pO2 but ↑pCO2 [Required Noninvasive ventilation (NIV) or Mechanical Ventilation is sometimes indicated immediately] [41].
Buffer system- it consists of weak acid or base and the salt of that acid or base and its function is to keeping pH in normal range (pH- dependent on HCO3-/H2CO3 Ratio). Bicarbonate is the most important buffer both intra and extracellular compartment. It is indicator of metabolic disorder. Respiratory processes alter pH by changing CO2 levels whereas Metabolic processes change pH by altering HCO3 content in blood.
In any disease process when serum HCO3- level less than 12 mEq/L should be corrected but in renal diseased condition it should be serum HCO3- level even less than 14 mEq/L. Note that patients hypo bicarbonatemia from renal failure cannot compensate from additional HCO3- loss from an external source (e.g. diarrhea) and severe metabolic acidosis can develop rapidly. In Acute Renal Injury where mild metabolic acidosis is common because of the retention of hydrogen ions, phosphate, and sulfate, but it rarely requires treatment. If acidosis is severe (arterial pH < 7.15; serum bicarbonate < 8 mEq/l) or contributes to significant hyperkalemia, treatment is indicated. The acidosis should be corrected partially by the intravenous route, generally giving enough bicarbonate to raise the arterial pH to 7.20 (which approximates a serum bicarbonate level of 12 mEq/L). The remainder of the correction may be accomplished by oral administration of sodium bicarbonate after normalization of the serum calcium and phosphorus levels. Correction of metabolic acidosis with intravenous bicarbonate can precipitate tetany in patients with renal failure as rapid correction of acidosis reduces the ionized calcium concentration [42].
In general, patients with renal failure tend to have a serum HCO3- level greater than 12 mEq/L and buffering by the skeleton prevents further decline in serum HCO3-. In Chronic Renal Failure, metabolic acidosis because of a decreased net acid excretion by the falling kidneys. Either Bicitra (1 mEq sodium citrate/ml) or sodium bicarbonate tablets (650 mg = 8 mEq of base) may be used to maintain the serum bicarbonate level22 mEq/L [43].
In significant metabolic academia, Alkali (NaHCO3-) should be given when the infant is receiving adequate assisted ventilation and pCO2 is in normal range. NaHCO3-, given when ventilation is inadequate, leads to respiratory acidosis and may worsen the patient’s condition. Tromethamine (THAM)→ can be used in infants who have a severe metabolic acidosis-may cause apnea because of its effect of rapidly lowering pCO2 [44].
Metabolic acidosis: Decrease in serum HCO3 (base) or gain of acid.
Diarrhea is one of the common pediatric disease manifested by purging with loss of fluid and valuable HCO3.
Diabetic Ketoacidosis is not uncommon in pediatric patients. It occurs in patients with insulin-dependent diabetes mellitus and is the result of severe insulin deficiency in the setting of increased metabolic demand, as would occur in the setting of a concurrent infection. As a result of insulin deficiency and depletion of glycogen stores, lipolysis ensues with increased production of ketoacids. Insulin is integral to the metabolism of ketoacids, and its relative or complete deficiency in the setting of increased ketoacid production results in severe keto-, i.e. AG acidosis.
Lactic Acidosisis commonly encountered in the pediatric ICU. It is caused by either increased lactate production or decreased hepatic metabolism. Tissue hypoxia secondary to hypotension with or without sepsis is the main cause. In sepsis, similar to other causes of circulatory failure, severe lactic acidosis, sets of a vicious cycle of further circulatory failure, worsening tissue perfusion, more lactate production and decreased consumption by the liver and kidneys [45-47]. In patients with severe status asthmaticus, lactic acidosis occurs secondary to increased work of breathing with elevated skeletal muscular oxygen demand.
Uremia while mild chronic renal failure can be associated with a non-AG, i.e. renal tubular acidosis (RTA), more advanced renal failure also results in an AG metabolic acidosis secondary to the accumulation of sulfates and other ions. Dialysis is the cornerstone intervention in treating this type of acidosis.
Inborn Error of Metabolism are rare genetic or inherited disorders resulting from enzyme defect in biochemical and metabolic pathways, usually present early in the neonatal life especially if severe affecting protein, carbohydrate, fat metabolism or impaired organelle function causing the body’s ability to turn food into energy or remove metabolic waste e.g. organic acidemias, maple syrup urine disease, urea cycle defects, fatty acid oxidation, aminoacidopathies. There is increased anion gap (due to increased acid pool) metabolic acidosis.
Metabolic alkalosis: Increase in serum HCO3 or loss of acid.
Main chemical compound in gastric content is HCL through vomiting but beyond the stomach it is HCO3- through diarrhea, gastro-cutaneus fistula or urethrogastro fistula.
NaHCO3 is a salt composed of a Na cation and a HCO3 anion. It might increase sodium levels in blood. So, patient who have already high levels of sodium in blood should avoid NaHCO3. In swelling (edema) as it contains Na increase the risk of swelling caused by excess fluids in the body. NaHCO3- might also lower K+ blood levels. So, patient already have low levels of K+ should avoid NaHCO3.
A plasma pH of 7.10 can be inconsequential when caused by diabetic ketoacidosis, but it portends a poorer outcome if it is secondary to septic shock and poor organ perfusion. Likewise, a plasma pH of 7.60 caused by anxiety-hyperventilation syndrome is inconsequential, whereas it signals a worse prognosis if it is secondary to a brain tumour [1].
Respiratory acidosis: Increased levels of CO2 due to decrease in lung ventilation leading to retention of CO2.
Respiratory alkalosis: Decreased levels of CO2 due to increase in respiratory-tidal volume leading to more elimination of CO2.
The patient one or more of these processes may be present. But both respiratory acidosis and alkalosis cannot exist together. Bronchopneumonia presented with respiratory acidosis, while in some patients with low albumin levels pointed out to the fact that respiratory acidosis may be compensated by metabolic alkalosis.
Fench., et al. on acid-base disorders in critically ill patients, found hypoalbuminemia, an almost ubiquitous abnormality in critically ill patients, can confound the interpretation of acid-base data when the customary diagnostic approaches based on BE or plasma [HCO3-] with AG are applied.BE fails as a measure of metabolic acidosis when the concentration of serum albumin, the main non-bicarbonate buffer in plasma, is low [48].
Treatment should center on re-establishing tissue perfusion.
Noninvasive through a mask or Invasive through an endotracheal intubation, mechanical ventilation or tracheostomy canula.
Mild→ No therapy required.
Improves with fluid and electrolyte balance is corrected.
Severe→ HCO3- < 12 mmol/L (Replacement is needed).
HCO3- mmol/L required= 0.35 × Body weight in kg × base deficit (mmol/l).
Half of the required dose→ diluting with equal volume of IV fluid can be given slow IV stat, Remaining Half can be put into the infusion bag to be infused over 6 - 8 hours.
In clinically suspected metabolic acidosis, empirically 1 - 2 mmol/kg IV, over 5 - 10 minutes may be given.
Monitor K+ level.
If NaHCO3 is given and the infant does not respond, think Inborn error of metabolism.
NaHCO3 in a cardiac arrest→ may cause harm and there is no evidence of benefit.
Do not treat metabolic acidosis with hyperventilation.
Treatment: Intravenous insulin is the most important therapy for patients with diabetic ketoacidosis [49]. Fluid, potassium, and phosphorous should be judiciously replaced. Insulin therapy induces the metabolism of ketones and results in the generation of alkali, hence obviating the need for sodium bicarbonate administration [50]. Indeed, sodium bicarbonate treatment that can delay the metabolic recovery by stimulating ketogenesis [51,52] was found to be of no benefit for patients with severe DKA (as defined by a pH of 6.9 - 7.14) and was associated with an increased risk of cerebral edema in children [53,54]. Therefore, sodium bicarbonate therapy is currently reserved for severe acidemia (pH < 6.9 in our practice) in order to avoid myocardial and cellular functional impairment at such an extremely low pH.
Management of lactic acidosis should center on reestablishing tissue perfusion while identifying and reversing the underlying disease [55-58]. Restoring intravascular volume and effective circulation is the cornerstone in treating patients with lactic acidosis and reversing the underlying cause promptly can be lifesaving. Specifically, management includes the provision of antibiotics in sepsis, operative repair of tissue ischemia or intestinal perforation, insulin for patients with diabetic ketoacidosis, congenital lactic acidosis [59]. Alkali therapy is not considered a standard intervention in lactic acidosis and carries a real risk of increased lactate production [60,61]. Nevertheless, it is our practice to utilize sodium bicarbonate in lactic acidosis when blood pH falls below 7.1, predominantly for concerns about hemodynamic compromise.
Fluid therapy for metabolic acidosis→ volume expansion should not be used to treat acidosis unless there are signs of hypovolemia. Severe acidosis causes a decrease in myocardial contractility.
Treatment of the primary cause.
Correction of dehydration with fluids containing adequate NaCl e.g.
Normal Saline.
KCl in infusion (K+ therapy) 3 - 6 mmol/kg/24 hrs.
Treatment of the primary cause.
Ventilation.
Re-breathing in a paper bag.
Treatment of the primary cause.
Respiratory arrest or repeated apnea (develops ↓O2 and/↑pCO2) → Requires immediate respiratory support. Ventilator issues → If the O2 level is high ↓ the FiO2. If the CO2 level is low ↓ the rate.
Septic shock (develops metabolic acidosis) → also may require respiratory support (e.g. mechanical ventilation), even if arterial blood gases are within acceptable range. Because patients need increased oxygen delivery to vital organs. Patient in shock often have respiratory distress due to metabolic acidosis, and the work of breathing and lactic acid production may be ameliorated by ventilator support.
Identification of the underlying cause or causes of the acid-base disorder at hand may be the final step in the management of sick children but it also plays an important role both in prevention of worsening of the derangement and other complications, as well as in the determination of the patient’s overall prognosis.
Copyright: © 2020 Mir Mohammad Yusuf. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.