Christian Chukwukere Ogoke1*, Ifeanyi Innocent Ike2, Wilson Chukwuneke Igwe3 and Edmund Ndudi Ossai4
1Department of Clinical Neurophysiology/Epilepsy Monitoring Unit, Mother Healthcare Diagnostics and Hospital, Owerri, Imo State Nigeria
2Department of Paediatrics, Federal Medical Centre, Owerri, Nigeria
3Department of Paediatrics, Nnamdi Azikiwe University, Nnewi Campus and Nnamdi Azikiwe University Teaching Hospital, Nnewi, Anambra State, Nigeria
4Department of Community Medicine, College of Health Sciences, Ebony State University, Abakiliki, Nigeria
*Corresponding Author: Christian Chukwukere Ogoke, Department of Clinical Neurophysiology/Epilepsy Monitoring Unit, Mother Healthcare Diagnostics, Nigeria.
Received: February 04, 2020; Published: March 06, 2020
Citation: Christian Chukwukere Ogoke., et al. “Clinico-Electroencephalographic Features of Children with Unprovoked Afebrile Seizures in Owerri, Southeast Nigeria”. Acta Scientific Paediatrics 3.4 (2020):02-08.
Background: Electroencephalography (EEG) plays a pivotal role in the diagnosis and management of epilepsies. Reports on pediatric EEGs are few in resource-poor settings due to the relative unavailability and inaccessibility of EEG facilities in these areas. Thus, only very few clinicians practicing in these settings are aware of the indications and limitations of EEG in the management of epileptic and non-epileptic paroxysmal events (NEPEs) in children. This study was therefore designed to ascertain the clinical and electroencephalographic profile of children referred for EEG in in Owerri, Southeast Nigeria.
Materials and Methods: This study was a retrospective review of consecutive records of all children who did EEG at a referral centre Mother Healthcare Diagnostics & Hospital Owerri, Southeast Nigeria between January 2017 and December 2018. Relevant data from each patient’s Basic Data Sheet and EEG findings were recorded.
Statistical analysis: Descriptive statistics was used in the analysis of results.
Results: Out of the 115 children referred for EEG, 70(60.9%) were males, 45(39.1%) females (M:F = 1.6:1). Fifty-six (49%) of these were under 5 years of age, 72 (62.5%) had a family history of epileptic seizures and 40(35%) were neurologically abnormal. Neonates were not referred for EEG during the period under review. Four (3.5%) were referred after first unprovoked seizure, 101(87.8%) after multiple episodes and 10 (8.7%) for NEPEs. Seizure disorder (unspecified) was the provisional diagnosis in 54(47%). EEG was normal in 47 (40.9%), abnormal in 56 (48.7%) and of poor quality in 12 (10.4%). The sensitivity and specificity of a first EEG in our cohort were 62.4% and 83.3% respectively.
Conclusions: Though half of the children utilizing EEG were under 5 years of age, no neonate was referred for EEG study. The majority of the children with epilepsy had a positive family history of epileptic seizures. Though recurrent convulsion was the most frequent reason for referral, there was little application of EEG after a first unprovoked afebrile seizure. EEG was found to be useful in our study in the management of children with epilepsy with modest sensitivity and high specificity.
Keywords: Electroencephalography; Children; Epilepsy
Electroencephalography (EEG) remains an invaluable investigative procedure in the diagnosis and management of epilepsies [1]. It is a recording of the electrical activity of the brain by means of electrodes placed on the scalp. EEG provides supportive evidence for the diagnosis of epilepsy, and also helps to distinguish focal-
onset from generalized-onset seizures. It is useful in identifying syndrome-specific changes that are useful in selecting appropriate anti-epileptic drugs and prognosis [1,2].
The sensitivity of an initial EEG is low because inter-ictal epileptiform abnormalities are found in less than 30% of patients [1].
The yield increases sharply to 80-90% with repeated recordings, increased duration of recording and appropriate activation procedures especially sleep [1]. Conversely, up to 1% of adults and 2-4% of children without clinical seizures have rigidly defined epileptiform abnormalities and 10% have nonspecific abnormalities [1].
Several factors account for errors in EEG. The most important source of error is interpreting the EEG out of clinical context [1,2]. This implies that the EEG record/ findings must be related to the clinical history and other laboratory data. Other factors include poor technical quality (equipment, personnel or both), overemphasis on non-specific EEG abnormalities without clarifying their significance, inability to recognize normal variants and evaluate age-related, physiologically-induced and drug-induced EEG changes [1,2]. Therefore, properly trained EEG technologists should record EEGs so as to obtain high-quality records and certified clinical neurophysiologists or Neurologists should interpret and report EEGs so as to make meaningful contribution to patient management.
There is a dearth of certified EEG technologists, clinical neurophysiologists and Child Neurologists in Nigeria with few EEG units serving the teeming population of patients with epilepsy and NEPEs. Although few studies in some parts of Nigeria have highlighted the relevance and application of EEG in patient management, none has been reported from South Eastern Nigeria [3-7]. Therefore, this present study set out to ascertain the profile of children referred for EEG, the pattern of findings and the sensitivity and specificity of a first EEG. The findings will provide information on the application of EEG in patient management and provide data on pediatric EEGs in our locality.
The study was carried out at the EEG unit of Child Neurology Clinic of Mother Healthcare Diagnostics & Hospital, Owerri, Imo state. Mother Healthcare Diagnostics & Hospital is a multi-specialist hospital and diagnostic centre with an EEG unit that was the first to commence pediatric digital EEG recording in Owerri, South east Nigeria. EEGs are done about 6 - 10 times per month and constitute less than 1% of the diagnostic services at the institution. The EEG laboratory is equipped with a portable digital EEG machine (NCC MEDICAL® , Nation 7128W/ Nation 7128WH {Type C EEG system}) and managed by a clinical neurophysiologist/ Child Neurologist. The EEG unit serves as the major centre for EEG for children seen at the Pediatric Neurology Unit of Federal Medical 03 Centre, Owerri (the only tertiary hospital in the capital city) and private and secondary care level hospitals in the state.
This was a retrospective review of consecutive medical records of all children under 18 yrs who presented for a 1st EEG study over 24 months period.
This research was approved by the Federal Medical Centre Owerri Health Research Ethics Committee (approval number: FMC/ OW/ HREC/ 232).
The cohort consisted of children aged < 18 years who were seen at the facility for their 1st EEG study between January 2017 and December 2018. To be included in the study, the Basic Data Sheet and other documentations must be complete. Those older than 18yrs of age or those for repeat EEG were excluded in the study.
The clinical history including information from pre-recording interview (Basic Data Sheet), EEG records and relevant data such as bio-demographic characteristics, sources of referral, clinical/preEEG seizure type diagnosis, EEG abnormalities, and post-EEG diagnosis were recorded in a data sheet. Information about ictal events, physical examination and other laboratory investigations such as ECG and neuroimaging obtained by the Clinical neurophysiologist during the preparation time for EEG were entered in the Basic Data Sheet used in this study. Each EEG was summarized as good or poor quality, normal or abnormal depending on the presence or absence of background abnormalities (disorganization, asymmetry/focal slowing, generalized slowing, excessive beta activity, background attenuation etc) or specific epileptiform abnormalities (spikes, polyspikes, sharp waves and their respective complexes). Post-EEG diagnosis was concluded by integrating the EEG findings with all relevant information (interpretation in a clinical context).
Data entry and analysis were done using IBM Statistical Package for Social Sciences (SPSS) statistical software version 25. Descriptive statistics was used in the analysis of results.
A total of 123 EEG recordings were done in children aged 18yrs and below during the period under review. Of this, the data of 8 leaving a study population of 115. This comprised of 70 males and 45 females (M:F= 1.6:1). The age range of the study population was 0.33 – 17.67 years, mean 5.88yrs SD ± 4.56 years. Fifty-six (48.7%) of the children were under 5 years of age while the least proportion (4.3%) was in the age group 15 - < 18 years. (Table I). Most of the referrals (76%) were from within the state capital while 28 (24%) were referred from outside the state capital. (Table II). The most common reason for referral for EEG was recurrent convulsion while only 4 (3.5%) were referred after a first episode of convulsion. (Table III). The majority of the patients (63%) had a positive family history of epileptic seizures while 25 (22%) had a history suggestive of severe birth asphyxia. A history of delayed development was obtained in 24% of cases while physical examination showed abnormal neurological state in 35% of cases. The other salient clinical features are shown in table 4.
Sleep was employed as an activation procedure in the majority (76%) of cases. EEG was of good quality in 90% of cases and uninterpretable in 10%. An abnormal EEG was obtained in 56 (48.7%) while the most frequent EEG background abnormality was hypsarrhythmia. The sensitivity and specificity of a first EEG for the cohort were 62% and 83.3% respectively. A summary of the main EEG findings is shown in table 4.
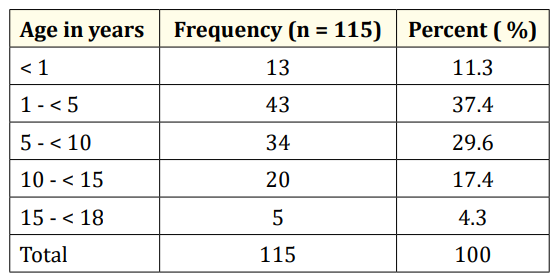
Table 1: Age distribution of children referred for EEG.
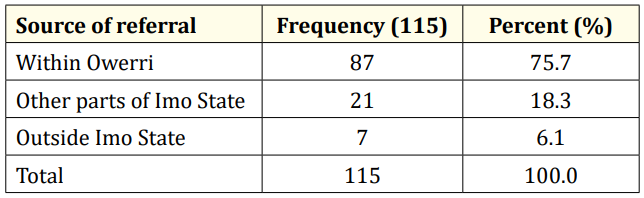
Table 2: Sources of referral.
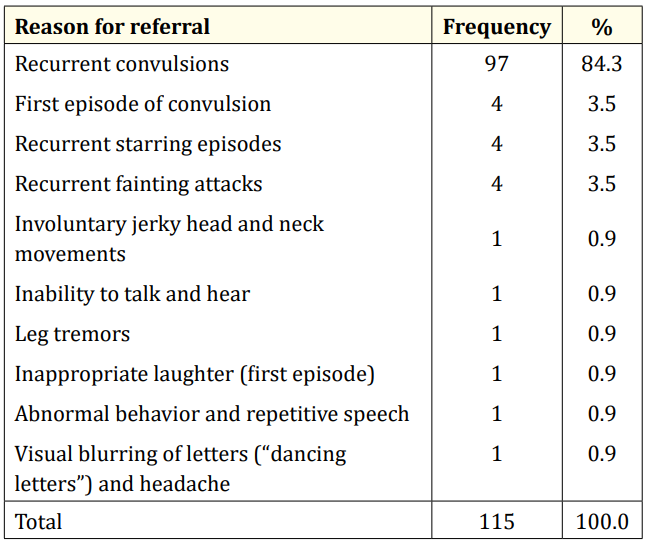
Table 3: Profile of reasons for EEG referral.
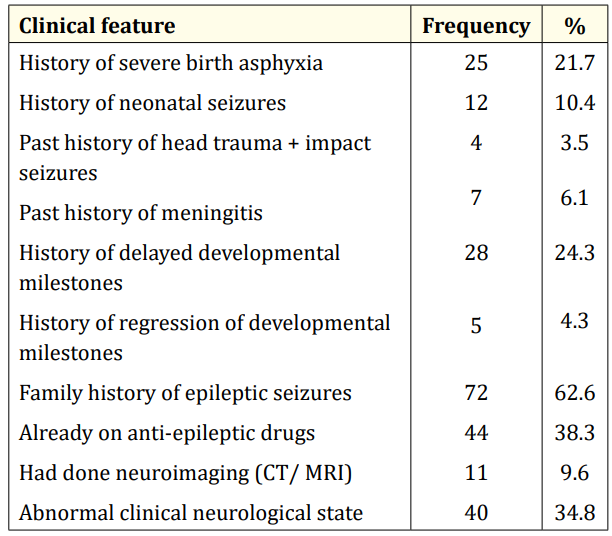
Table 4: Salient clinical features of the study population.
An EEG is a simple noninvasive investigative tool for recording and evaluating the paroxysmal hypersynchronous discharges of cerebral neurons that cause seizures [8]. Thus, all patients with epileptic seizures need an EEG due to its critical contribution to the diagnosis and management of epilepsies and NEPEs. This present study has shown that although half of the patients referred for EEG were under five years of age, no neonate was referred for EEG during the period under review. The implication of this finding is that there is little or no application of EEGs in the management of neonates in our locality since it is well known that seizures are highly prevalent in the neonatal period and ictal and inter-ictal EEGs are required for accurate diagnosis and prognostication. Possibly, the clinicians in our locality may not be aware of the significance of neonatal EEGs regardless of its limitations. Therefore, pediatricians and other clinicians may need to be enlightened to request an EEG in neonates with suspicious repetitive and stereotypical events.
Studies in Nigeria have shown that pediatric neurologic disorders are more common amongst males and in the younger age groups [9-12]. Epilepsy, cerebral palsy and central nervous system infections constitute the majority of these neurologic disorders [912]. The age distribution and male preponderance reported in this study are similar to the findings by Aina., et al [13] in South-West Nigeria. About half of the children referred for EEG were under five years (U5s), but one realizes that the proportion of U5s in the cohort would have been higher if neonates were also referred.
Less than a quarter of the respondents (24%) had to travel hundreds of kilometers from outside the state capital and neighbouring states to access EEG. This depicts the relative unavailability and poor accessibility of neurodiagnostic tests in resource-poor settings. The total number of EEGs done in children (123) in two years is also an evidence of poor utilization. There are insufficient EEG units for the teeming population of patients with epilepsy and non-epileptic paroxysmal events (NEPEs) in Nigeria and thus leading to a “diagnostic gap” and inadequate management. Mclane., et al [14] had earlier reported the unavailability, inaccessibility and unaffordability of neurodiagnostic tests in developing countries [14]. In the present study, only 10% of the patients had neuroimaging studies (CT/MRI) even though one out of every three of the patients (35%) presented with hard neurological signs. Therefore, there is need for the provision of well-equipped neurophysiology laboratories and neuroimaging equipment in all federal and state hospitals in Nigeria.
Most referrals for EEG are to investigate suspected epilepsies in patients with recurrent seizures and this was evident in our findings. Since an EEG in the untreated stage of an epilepsy syndrome is imperative and best recorded after the first unprovoked seizure, the Child Neurology Society and American Epilepsy Society recommend obtaining a routine EEG after a first afebrile seizure [1,15] In addition, according to the most recent/ updated definition of epilepsy by Fisher., et al, [16] an EEG after a first unprovoked seizure is of diagnostic relevance since the documentation of hypersynchronous activity or epileptiform potentials also constitutes epilepsy. We found that very few (3.5%) of our patients had an EEG after the first episode of unprovoked seizure. This implies that General physicians, General Paediatricians, neurologists and other healthcare workers in Nigeria should be enlightened on the reasons for requesting an EEG after the first unprovoked afebrile seizure. The rationale is that an epileptiform activity in EEG after a first unprovoked seizure translates to ≥ 60% risk of recurrence over the next 10 years and is similar to the general recurrence risk after two unprovoked seizures that is traditionally used to make a decision to treat [16]. The positive family history of epilepsy in the majority of the patients (63%) suggests a likelihood of predominance of genetic epilepsies or genetic etiology in those with epilepsy.
Obtaining a good quality EEG in children depends on the technician’s skills and experience in handling children including an awake and sleeping infant. Despite the technical difficulties of performing EEG in younger children, 90% of our records were of good quality (Table 5). In our experience, tabs melatonin (3 – 6mg <15kg, 6 - 9mg >15kg ) was very useful in producing the needed sedation in most cases with less movement artifacts on EEG and no postsedation irritability or persistent drowsiness after the procedure. This may also have implications for the diagnostic yield of the EEG in our cohort who all had a first EEG since an EEG in drowsiness, sleep and awakening is known to increase the diagnostic yield of certain epileptiform patterns [1]. Drug-induced sleep was an additional activation procedure in 76% of the patients and may have augmented the proportion of epileptiform EEGs. Ibekwe., et al [17] had earlier reported the effectiveness and safety of melatonin in inducing sleep for EEG recording in sub-saharan Africa.
Inter-ictal epileptiform abnormalities are usually detected in less than 30% of an initial EEGs but the yield increases to 80 – 90% with repeated EEGs, prolonged recording and sleep EEG [1]. Moreover, the reported sensitivities and specificities of EEG vary widely between studies (23 – 77% & 24 – 99% respectively) [19-20]. Furthermore, the occurrence of abnormalities on EEG has been shown to be higher in children than adults [19]. This present study found abnormalities in 48.7% of cases (Table 5) which is higher than that reported in a similar study by Bozoro., et al [21] However this was amongst adults and a larger sample size and larger statistical power. The proportion of EEG abnormalities in this present study is, however, lower than 76.6% reported by Aina., et al [13] in a cohort of children with neurodevelopmental disorders. This is explained by the difference in the profile of the two cohorts since there is a significantly higher prevalence of epileptiform abnormalities in children with neurodevelopmental disorders irrespective of the occurrence of clinical seizures [1]. Lagunju., et al [7] in their study of 329 children also reported a much higher proportion ( 94%) of abnormal EEGs than the present study. It is difficult to explain the wide difference in findings except considering repeated records or “overinterpretation” since initial EEGs are not known to be very sensitive.
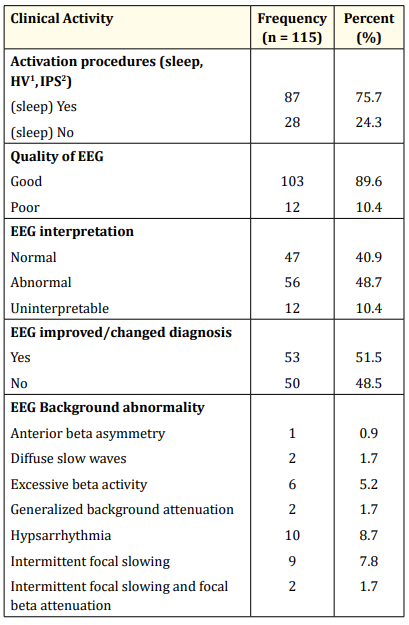
Table 5: Summary of EEG findings. HV1 : Hyperventilation; IPS2 : Intermittent Photic Stimulation.
We found a moderate sensitivity (62%) but high specificity (83%) for a first EEG and a positive predictive value (95%) much higher than the negative predictive (32%) (Table 6). It has been reported that higher specificity EEG readers have a greater accuracy in predicting seizure recurrence than did higher sensitivity readers [20].
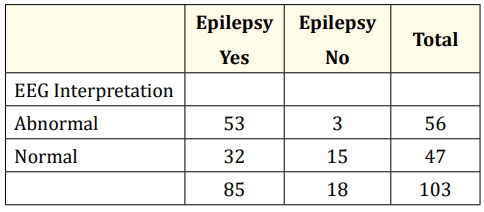
Table 6: Sensitivity and specificity of EEG (n = 103). Sensitivity = 53/85 = 62.4%; Specificity 15/18 = 83.3% Positive Predictive Value = 53/56= 94.6%; Negative Predictive Value = 15/47=31.9%
This suggests that the patients in our study who had a negative EEG are less likely to have another seizure episode. This finding has thus highlighted one of the important uses of EEG after a first unprovoked afebrile seizure because it helps to predict the risk of seizure recurrence since an epileptiform EEG is associated with determining a two- to three times higher risk of seizure recurrence than a normal EEG [1]. However, 84% of the patients referred for EEG had documented recurrent convulsions (Table 3) and are therefore expected to have ongoing recurrent seizures.
The most frequent EEG background abnormality (hypsarrhythmia) seen in this present study suggests that the epileptic encephalopathy called West syndrome was prevalent in the cohort. Lagunju., et al [22] reported the high prevalence of symptomatic epilepsy in Nigerian children resulting from preventable conditions such as perinatal asphyxia and intracranial infections. However, our finding does not imply that West syndrome was the commonest epilepsy syndrome in the cohort (Table 5) since the more common benign childhood seizure susceptibility syndromes like Benign Rolandic Epilepsy (BRE) and Panayiotopoulos syndrome belonged to the number that had normal background activity and this was more than twice the total number with abnormal background activity. Some of those EEGs with normal background were eventually interpreted as abnormal due to presence of epileptiform abnormalities.
The retrospective design of this study is one limitation due to missing clinical information in some cases. In addition, the small sample size of our study may limit drawing general conclusions.
Though half of the children utilizing EEG were under 5 years of age, no neonate was referred for EEG. The majority of the children with epilepsy had a positive family history of epileptic seizures. Though recurrent convulsion was the most frequent reason for referral, there was little application of EEG after a first unprovoked afebrile seizure. EEG was found to be useful in our study with modest sensitivity, high specificity and a high level of accuracy in predicting risk of seizure recurrence especially for those patients with a first unprovoked afebrile seizure.
None.
None.
Copyright: © 2020 Christian Chukwukere Ogoke., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.