Rashmi Patil*, Megha, Chikkanarasareddy PS, Panikaj AT, Vijeta V Rane and Sarala Sabapathy
Department of Paediatrics, Bangalore Medical College and Research Institute, Bangalore, India
*Corresponding Author: Rashmi Patil, Department of Paediatrics, Bangalore Medical College and Research Institute, Bangalore, India.
Received: January 21, 2020; Published: February 06, 2020
Citation: Rashmi Patil., et al. “Accidental Ingestion of Dapsone in Children - A Case Report Experienced in Government Tertiary Care Hospital”. Acta Scientific Paediatrics 3.3 (2020):09-12.
Dapsone, a sulfonamide derivative is being increasingly used in the treatment of a wide variety of dermatological disorders. Dapsone intoxication in children is most commonly, accidental and uncommon. The most frequent reaction that occur with higher doses of Dapsone toxicity is hemolytic anemia and methemoglobinemia. Rapid clinical assessment, measurement of methemoglobin levels and meticulous institution of treatment with Intravenous methylene blue decreases the mortality and morbidity. Here we report a 4 years old girl with methemoglobinemia secondary to severe dapsone poisoning with sinus arrhythmia and its successful management.
Keywords: Accidental; Dapsone Consumption; Children; Methemoglobinemia; Sinus Arrhythmia; Methylene Blue
Dapsone (4, 4-diaminodiphenylsulfone), a sulfonamide derivative, was introduced in 1943 as an effective chemotherapeutic agent for leprosy [1-3]. Dapsone is increasingly used in the treatment of a wide variety of dermatological disorders in tropical world [4,5]. Because of its use in various conditions, its toxicity is commonly seen in adults [4]. However, dapsone intoxication in children is usually accidental and has been considered invariably fatal [6,7]. Methemoglobinemia is the major life-threatening situation associated with poisoning of DDS [8]. In initial stages of acute poisoning, there will not be any major manifestations and hence there may be delay in seeking medical attention [9]. Here we report a case of accidental severe dapsone poisoning with methemoglobinemia with sinus arrhythmia.
A four year old girl was brought to the paediatric casualty with complaints of accidental ingestion of 20 tablets of 100mg each dapsone tablets. Dapsone tablets were taken by her uncle for dermatitis herpetiformis. The child presented with persistent vomiting, lethargy, drowsiness, unsteadiness and bluish discoloration of lips and oral cavity, seven hours post consumption. At admission child was drowsy with vacant stares. Patient had central and peripheral cyanosis. Euthermic with tachycardia [HR180bpm], tachypnea [RR52cpm] BP:104/68mmHg (50-90th centiles), oxygen saturation (SPO2) was 65% under room air with arterial blood gas analysis showing (pH: 7.45, PCO2: 21.4 mmHg, paO2: 102 mmHg) base deficit- 8.
Stomach wash was given with activated charcoal (15gm), IV ascorbic of 500mg was given and Methylene blue 2 mg/kg (15mg) was administered, slow IV and by one hour SPO2has improved to 94%. By six hours SPO2 dropped to 75% and second dose of methylene blue given, post which SPO2 has maintained at 83% with oxygen (O2). On day three of admission, child again had desaturation SPO2 reached 75%. Third dose of IV Methylene blue (Total of 6mg/ kg given). Same day child was noticed to have sinus arrhythmia, Electro cardiac monitoring was continued. Troponin-I negative, ProBNP 594pg/ml. Sinus arrhythmia settled in a day; taken as sick nodal dysfunction secondary to hypoxemia. Evidence of hemolysis characterized by drop in hemoglobin, hematocrit and increase in reticulocyte count (3.6%) was observed on day four of admission for which PRBC transfusion was given. Next two days, we have noticed ataxia. There was no derangement of renal or liver function tests, urinalysis found to be normal. G6PD levels 111.2 units/ dL within normal limits. Hemoglobin electrophoresis was normal. Methemoglobin levels could not be assessed, not done in our setting. In the mean time, Gastric lavage, blood and urine samples sent for Forensic Lab confirmed presence of Dapsone. On day seven, child was maintaining SPO2 85-89% with room air, no neurological deficits. Child was discharged at the end of two weeks. During follow up period, child had no cyanosis with saturation of 95-97% and neurological examination falls within the normal limits.

Figure 1: Dapsone tablet IP.
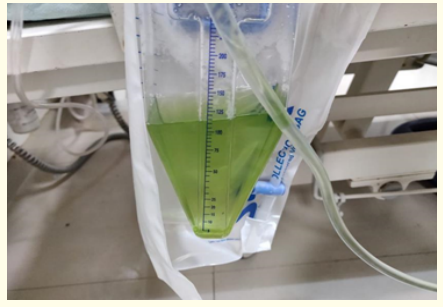
Figure 2: Urine turned green after treatment IV Methylene blue.
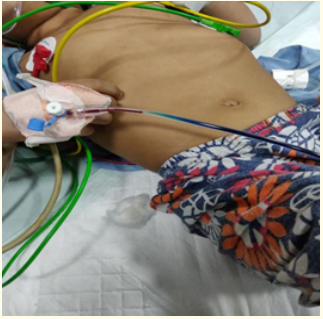
Figure 3: On flow IV methylene blue.

Figure 4: At the time of admission.
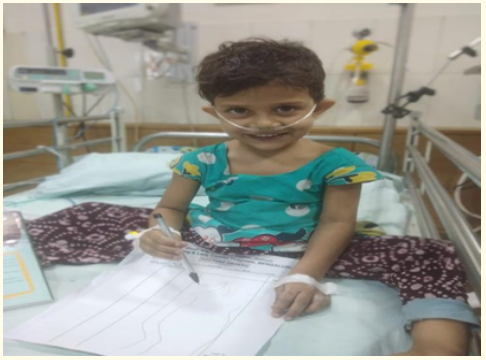
Figure 5: Final day of discharge.
Dapsone, a sulfone is a structural analog of paraaminobenzoic acid (PABA) and a competitive inhibitor of dihydropteroate synthase in the folate pathway [7]. The indications for dapsone are varied and include the management of Pneumocystis carinii pneumonia (PCP) in immunosuppressed patients, dermatitis vulgaris, herpetiformis, psoriasis, pemphigus, acne lupus erythematosus profundus, brown recluse spider bites, pyoderma gangrenous [7] prophylaxis and treatment of falciparum malaria maduromycosis, panniculitis due to alpha-1 antitrypsin deficiency [1,4,9]. Accidental dapsone poisoning in children is uncommon [8]. The poisoning may present with severe clinical symptoms like cyanosis, nausea, vomiting, headache, respiratory difficulty, drowsiness and abnormal body movements [10]. The CNS manifestations observed are irritability, hypotonia, truncal ataxia, choreiform movements and dysarthritic speech. In our case, child had cyanosis, nausea, vomiting, drowsiness, hypotonia and truncal ataxia. The plasma elimination half-life of Dapsone was found to be dose dependent which varies from 10 to 80h (mean 30h), peak levels after two to six hours [11]. The drug can be detected in tissues up to three weeks after ingestion [12]. The renal excretion of unchanged Dapsone is limited to approximately 20% of the administered dose. Dapsone is metabolized in the liver for its elimination. It undergoes N-acetylation by N-Acetyltransferase (NAT2). Dapsone hydroxylamine enters red cells leading to methemoglobin formation. The most frequent reaction that occur with higher doses of Dapsone toxicity is hemolytic anemia and methemoglobinemia [9-12]. The N-hydroxylation of dapsone to its hydroxylamine metabolite is in part responsible for methemoglobinemia in both therapeutic use and over dose. Many scientist reported that, decrease in hemoglobin (1-2 g/dL) and reticulocyte count (2%-12%) levels in patient with Dapsone toxicity [13]. Clinical severity depends on blood Methemoglobin levels [13,14]. Methemoglobin is an oxidation product of Hb in which there is an oxidized ferric iron in sixth co-ordination position instead of reduced ferrous iron in normal Hb. This oxidized ferric iron containing site is then bound to a water molecule or to a hydroxyl group [14]. This complex is dark brown and unable to transport oxygen with a leftward shift in oxygen dissociation curve, thus leading to a decreased tissue oxygenation with subsequent hypoxic features. This also explains why the delivery of oxygen to patient does not improve the oxygen saturation level. Most of the patients are found to be asymptomatic until approximately 30% of hemoglobin is presented as Methemoglobin [15]. However, levels especially greater than 15% may be associated with cyanosis. High mortality rate is associated with levels above 70% [16].
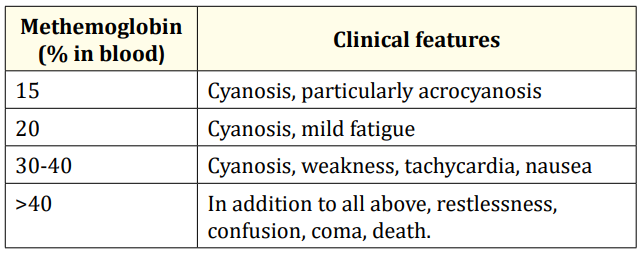
Table 1: Clinical features of dapsone poisoning is determined by methemoglobin level (%) in blood.
This child had lethargy, tachycardia and Dizziness corresponding to Methemoglobin between 30-40%. Level of Methemoglobin above 2% is abnormal [9]. According to a Brazilian study conducted by Maria Zilda N., et al. Dapsonemia was considered severe when 20 tablets (100 mg each) were ingested, a median of 29 µg/ ml. Regarding Methemoglobinemia, intoxication was severe when 7.5 tablets were ingested, a median of 38% of the total Hb [10]. In our case, child had accidentally consumed 20 tablets of Dapsone 100mg each and hence is Severe Dapsone poisoning. In this case, excessive levels of dapsone may have led to corresponding decrease in hemoglobin levels. Acute hemolytic anemia in dapsone intoxication as observed in the present case may occur due either to the continued oxidative stress by the dapsone metabolites or bolus doses of methylene blue [12-18]. Thus, children should be observed for hemolysis by repeated hematocrit estimation and peripheral blood smear examination and need to be treated with packed cell transfusion, if needed. The standard management of orally ingested drug induced methemoglobinemia includes gastric lavage, administration of activated charcoal (0.5-1g/kg), methylene blue and ascorbic acid. Methylene blue is the drug of choice in the treatment of methemoglobinemia [9]. Methylene blue is a phenothiazine-related heterocyclic aromatic molecule most commonly used as a reducing agent in the treatment of methemoglobinemia and for the treatment of cyanide and carbon monoxide poisoning [19]. Methylene blue (methylthioninium chloride) when given intravenously is rapidly reduced to leuco-methylene blue by the enzyme NADPH-met-Hb reductase which reduces the met Hb back to Hb by a cyclic reaction [4,20]. Conventionally, methylene blue has been given intermittently every 6–8 hours as needed during prolonged dapsone intoxications. The dosing of methylene blue is not entirely clear, but 1-2 mg/kg is used for the treatment of methemoglobinemia. However, methylene blue above 7 mg/kg is associated with adverse effects such as paradoxical induction of Methemoglobinemia Conversely, because methylene blue is an oxidizing agent, it can itself cause methemoglobinemia by oxidizing hemoglobin at high concentrations hemolytic anemia and detrimental effects on pulmonary function [21,22]. Therefore, methylene blue should not be recommended in patients with pulmonary hypertension, underlying glucose-6-phosphate dehydrogenase deficiency and acute lung injury [22]. Concurrent use of other innocuous reducing agents with methylene blue such as ascorbic acid is known to compete directly with the chemical cause and also to provide a reducing environment in blood to allow the dye to act more efficiently [4]. In less severe cases, ascorbic acid 200-500 mg can be given intravenously as was used in our case [23]. In a case reported by Agrawal., et al, Ascorbic acid alone was used successfully to treat Methemoglobinemia due to Dapsone consumption [7]. Treatment with methylene blue can be complicated by the presence of underlying glucose-6-phosphate dehydrogenase deficiency alternative therapies like exchange transfusions and hyperbaric oxygen therapy are the remaining options in patients with glucose6-phosphate dehydrogenase deficiency or if methylene blue therapy is ineffective [24]. The exchange transfusion had been reported to be very effective in the management of methemoglobinemia [25] but was later found to be of minor benefit, probably due to large volume of distribution of dapsone [26]. Charcoal hemoperfusion has also been reported for the rapid clearing of dapsone [27]. In the presence of the increased Methemoglobin fraction, pulse oximeter values will trend toward 85% underestimating the actual oxygen saturation [9]. Routine pulse oximetry is generally inaccurate for monitoring oxygen saturation in the presence of methemoglobinemia as a saturation gap exists [28]. In our case, child had sinus arrhythmia; none of the previously reported cases had arrhythmia.
Any case presenting to emergency department with cyanosis and breathlessness not responding to oxygen must be suspected as a case of methaemoglobinemia. Dapsone a commonly used drug in the treatment of varied dermatological conditions; should be kept out of reach of children to prevent significant morbidity and mortality. In spite of adequate treatment, recovery can take weeks.
Copyright: © 2020 Rashmi Patil., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.