Archana S*, G Shiva Krishna, Vijalakshmi, Devika Gunasheela
Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai, India
*Corresponding Author: Archana S, Research Scholar, Department of Human Genetics, Faculty of Biomedical Sciences, Technology and Research, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai, India.
Received: December 13, 2019; Published: January 06, 2020
Citation: Archana S., et al. “Pregnancy Outcome in Fresh and Frozen Embryo Transfer in Women with High Estradiol Levels”. Acta Scientific Paediatrics 3.2 (2020):03-06.
Exogenous elevation of serum estradiol shortly after the time of ovulation is known to reduce the endometrial receptivity in natural cycle. High serumestradiol may affect the synthesis and secretion of glycogen by endometrial epithelial cells. Studies on endometrial morphology, biochemistry and endometrial genomic pattern at the time of implantation showed that high estradiol may negatively affect the endometrial receptivity during infertility treatment. Highestradiol is one of the contributing factors for OHSS.
Controlled Ovarian Hyperstimulation (COH) creates a supraphysiologic environment which affects endometrial receptivity. Frozen embryo transfer avoids supraphysiological environment which alters the endometrial receptivity. Endometrial development can be controlled precisely in frozen embryo transfer cycle than in COH with gonadotropins.
Keywords: Pregnancy; Estradiol; Frozen Embryo Transfer
High estradiol levels showed lower implantation and pregnancy rates (Forman., et al. 1988; Simon., et al. 1995) where as some other studies (Chenette., et al. 1990) have showed no adverse effects. Elevation of estradiol levels during the First trimester may lead to impaired angiogenesis and results in abnormal placentation.
To compare pregnancy outcomes in women with estradiol levels greater than 2500pg/ml and number of oocytes obtained ≥14.
Retrospective study conducted at Gunasheela IVF centre from 2011 to 2013. 122 patients were meeting the study criteria. All the patients were ≤ 35years old. Total number of oocytes obtained per patient ≥14 and Estradiol levels on the day of HCG ≥ 2500pg/ ml were the inclusion criteria. Oocyte Donor/recipient cases and Intracytoplasmic sperm injection with donor spermatozoa were excluded. Two groups were included in the study. Group I had 79 patients who had undergone fresh embryo transfer. Group II had 43 patients who had undergone frozen embryo transfer (FET) with no fresh embryo transfer. Fresh embryo transfer was cancelled in group II patients due fluid collection >70cc showed in scan in 32 patients and 11 patients had lupride trigger. All the patients in group II had PCOS. Patients in group II was diagnosed as PCOS based on ultrasound findings. Fresh embryo transfer was cancelled in group II due to risk of OHSS. Controlled ovarian stimulation using gonadotropins was done either along with agonist or antagonist protocol. HCG trigger was given when the leading follicle size was ≥18mm for all the patients in group I and 32patients in group II.11 patients in group II had lupride trigger (leuprolide acetate 1mg). Oocyte retrieval was done 34-36hours after HCG injection. Estradiol levels were checked on the day of HCG in all the patients. In Group II, patients had stimulation by straight HRT (hormone replacement Therapy) or Luteal phase down regulation HRT (LPDR+HRT) in Frozen embryo transfer cycle. Ovral-L (levonorgestrel + ethinylestradiol) was given from Day5-day25 in LPDR+HRT for down regulation and followed by administrating GnRH agonist (Lupride) 1/2mg from Day 21 of previous cycle. GnRH agonist (Lupride) was reduced to 1/4mg from Day2/Day 3 of next cycle and Hormone replacement therapy was started with an initial dose of Estradiolvalerate (Progynova) 2mg and increased by monitoring endometrial measurement. In straight HRT cycle, Estradiolvalerate (Progynova) 2mg was started from Day3 of the cycle and increased according to the endometrial measurement. FET patients had estimated their estradiol levels when the scan showed tripe line pattern of the endometrium.
Vitrification procedure was done by subjecting the embryos to Equilibration solution for 8-15minutes and vitrification solution for 90-110seconds respectively. Warming procedure was done by subjecting the embryos to 1Molar solution for 1min, 0.5Molar solution for 4min and HEPES solution for 9min respectively.
Statistical analysis was done with chi-square test. Biochemical pregnancy rate, Clinical pregnancy rate, Implantation rate and live birth rate was compared in both groups. P-value <0.05 is considered as statistically significant.
Average age of the patients was 29.6 ± 3.9years and Group II was 30.2 ± 3.1 years. 1469 oocytes were retrieved in Group I and 606 oocytes were retrieved in Group II.975 mature oocytes obtained at 0hrs,184immature oocytes had matured after 24hrs culture in in vitro-maturation (IVM) media (sage) in Group I. Maturation rate was 38.25% (184/481) in Group I.524 mature oocytes obtained at 0hrs,30 oocytes had matured after 24hrs in IVM media in Group II. Maturation rate in Group II was 37.5% (30/82).Total number of oocytes in Group I was 1159 and total number of oocytes in Group II was 554.Fertilisation rate was 83% (814/975) in Group I whereas fertilization rate in Group II was 75.81% (420/554).Cleavage rate was 96% (784/814) in Group I whereas cleavage rate was 97.61% (410/420) in Group II as shown in Table 1.
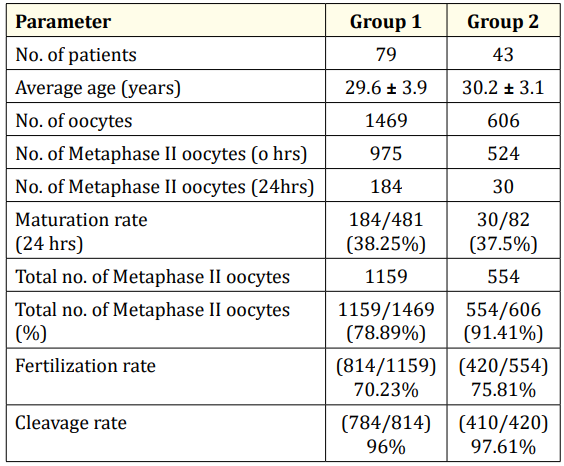
Table 1
181 embryos were warmed from 43 patients who had undergone frozen embryo transfer. Embryo survival rate was 72.37% (131/181) as shown in table 2.

Table 2
Estradiol levels in Group I was 2795 ± 914.1pg/ml at the time of HCG. Estradiol levels at the time of HCG or lupride trigger was 2984 ± 375.3pg/ml and at the time of transfer of frozen thawed embryos was 309 ± 175pg/ml in group II patients. Average number of embryos transferred per patient in Group I and Group II was 2.7 and 2.6 respectively as shown in table 3.
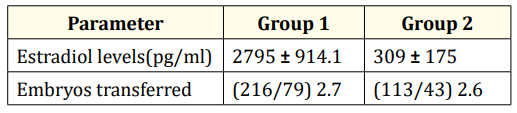
Table 3
Total pregnancy rate in Group I and Group II was 40.5% and 55.8% (p=0.105) respectively. Biochemical pregnancy is defined as pregnancy diagnosed by detection of HCG in serum or urine and that does not develop into a clinical pregnancy. Biochemical pregnancy rate in Group I and Group II was 40.5% and 55.8% (p= 0.105) respectively. Pregnancy diagnosed by ultrasonographic or clinical documentation of at least one foetus with heartbeat is defined as Clinical pregnancy. Clinical pregnancy rate in Group I and Group II was 36.7% and 48.8% (p = 0.19) respectively. Implantation rate in Group I and Group II was 22.2% and 18.8% (p = 0.44). Live birth rate in Group I was 20.25% and in Group II was 44% (0.005) as shown in table 4.
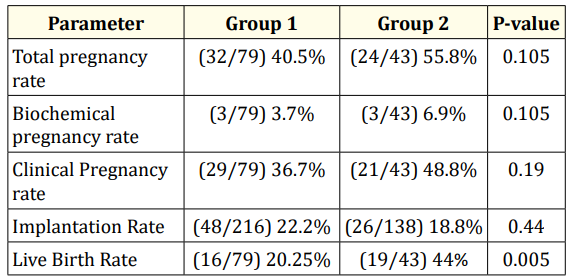
Table 4
This study showed high estradiol levels had an effect on live birth- rate. Estradiol levels for patients who had undergone fresh embryo transfer were 2795 ± 914.1pg/ml. They had showed a live birth-rate of 20.25% (16/79). Frozen embryo transfer had a live birth-rate of 44% (19/43) and estradiol levels were 309 ± 175pg/ ml. Total pregnancy rate, clinical pregnancy rate, Implantation rate didn’t showed much difference in both groups. Implantation rate in Group I and Group II were 22.2% and 18.8% respectively. Live birth rate is statistically significant (p<0.005). There is a drastic fall in live birth rate in fresh embryo transfer when compared to frozen embryo transfer [1-26].
Decrease in live birth-rate may be due to abnormal placentation. Altered trophoblastic invasion into myometrial and uterine vessels leads to abnormal placentation and results in activation of maternal vasoconstrictors.
Ovarian stimulation leads to elevated estrogen levels which may affect the endometrium. High estradiol creates a supraphysiological environment which results in decreased trophoblastic invasion of the decidual and myometrial spiral arteries, aberrant cell survival and apoptosis which leads to abnormal placentation. The ultimate consequence of this phenomenon is that these arteries retain the ability to respond to vasoactive stimuli, causing incessant vasoconstriction. During the course of pregnancy, vasoconstriction may lead to suboptimal blood supply to the growing placenta and subsequent spontaneous abortion, stillbirth, small for gestational age (SGA), orpreclampsia (PreE).
During the formation of normal placenta, Trophoblast divides into villous and extra-villous trophoblast after implantation. Cytotrophoblast which acts as a source of proliferative cells gives rise to syncytiotrophoblast and extra villous trophoblast. Syncytiotrophoblast in contact with maternal blood gives rise to villi. Extra-villous trophoblast segregates into trophoblastic shell, interstitial and endovascular cells respectively invading decidua, myometrium and uterine vessels.
Invasion of the trophoblast into maternal tissue leads to remodelling of arterial walls with loss of smooth muscle and associated elastic and collagenous extracellular mass. They will be replaced by unique fibrin based polymeric deposit (fibrinoid). This results in conversion of high resistance low capacity vessels into low resistance high capacity vessels and made independent of maternal vasoconstrictors in normal placentation. Activation of these maternal vasoconstrictors takes place in abnormal placentation which results in spontaneous abortion, stillbirth, SGA or pre E and affects the live birth-rate.
In Conclusion, Frozen embryo transfer is better than fresh embryo transfer in women with estradiol levels greater than 2500pg/ ml and total number of oocytes obtained greater than 14.
Copyright: © 2020 Archana S., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.