Fadi Rayya1* and Turki Alhasoon2
1Department of General Surgery, Al Assad University Hospital, Faculty of
2General Surgery, Faculty of Medicine, Damascus University, Damascus, Syria
*Corresponding Author: Fadi Rayya, Consultant Surgeon, Department of General Surgery, Al Assad University Hospital, Faculty of Medicine, Damascus University, Syria.
Received: November 28, 2019; Published: December 26, 2019
Citation: Fadi Rayya and Turki Alhasoon. “Resection of Recurrent Hepatoblastoma: A Case Report”. Acta Scientific Paediatrics 3.1 (2020):36-38.
Hepatoblastoma (HB) is considered to be one of the most common liver malignant tumors in children. A three years old male presented with a hepatic mass, which was diagnosed as recurrent Hepatoblastoma. After neoadjuvant chemotherapy the patient underwent a successful hepatectomy.
Keywords: Hepatoblastoma; Liver; Chemotherapy
Hepatoblastoma (HB) is the most common primary liver tumor in children, approximately 90% of the cases occur under the age of 5 years [1].
It forms two-thirds of malignant liver tumors [2], and consists 1-4% of all cases amongst all primary malignant tumors in children [3]. Because of its rarity and inherent malignant nature, diagnosis and treatment is problematic [4].
The primary treatment is surgical resection, however, chemotherapy plays an important, role in downstaging and converting the nonresectable to resectable tumors. Prognosis for patients with resectable tumors is fairly good. The survival of patients with a hepatoblastoma has markedly improved in recent years with the advances in chemotherapy and surgery.
Several national and international cooperative studies have shown that the prognosis for hepatoblastoma can be improved dramatically by combining surgery with pre- and post-operative chemotherapeutic agents such as cisplatin and adriamycin [5,6].
Complete resection of hepatoblastoma in combination with neoadjuvant and/or adjuvant chemotherapy has improved overall survival for patients with this pediatric solid tumor from approximately 30% in the 1970s to 70-90% in the modern era [7].
Hepatoblastoma rarely spread to other areas. However, features such as metastases at diagnosis, low expression of alphafetoprotein (AFP) and multifocal disease significantly reduce survival [7,8].
Recurrent HB is rare, it usually combines with the rise of AFP.
A three-year-old boy presented with an enlargement of the abdomen and decreased appetite without any other symptoms.
Clinical examination demonstrated pallor but no jaundice, enlargement of abdomen, no Medusa sign, palpable hepatomegaly of 4 cm below the costal border, without a splenomegaly or an ascites. The patient underwent previous surgery due to hepatic HB since fifteen months.
Blood investigations were normal except a low haemoglobin of 7.7mg\dl (normal range >11mg\dl) and AFP was elevated; it was >1200ng/mL (reference range 0–15ng/mL).
An abdominal ultrasound revealed a large mass, extending inferiorly from the right lobe of the liver and measuring approximately (4 x 5)cm.
Thoracic and abdominal CT scan showed a large heterogeneous hemolytic lesion fills the right lobe of the liver and contained multiple necrotic foci and coarse and scattered calcifications, this mass showed a compressing effect of the portal vasculature (6 x 7) cm (Figure 1).
Laboratory and radiological findings indicate a recurrent HB.
A work-up including traditional scintigraphy revealed no metastatic disease. Neoadjuvant chemotherapy was applied, but the size of tumor got bigger. We prepared the patient to have surgery.
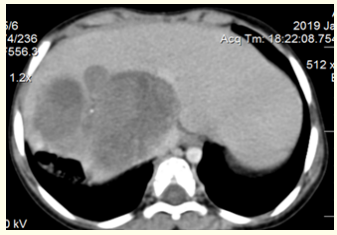
Figure 1: Abdomen CT scan shows the tumor and its relationships with the VCI.
We performed an extended right hepatectomy in which we isolated and dissected the tumor from Inferior vena cava(IVC), the tumor was completely resected, although, it was adjacent to the IVC, there was no need for blood transfusion during surgery (Figure 2,3).
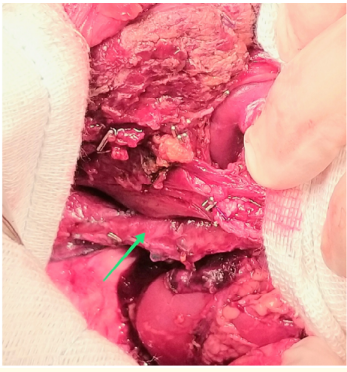
Figure 2: Intraoperative view demonstrates the remnant liver and VCI (the arrow) after right hepatectomy.
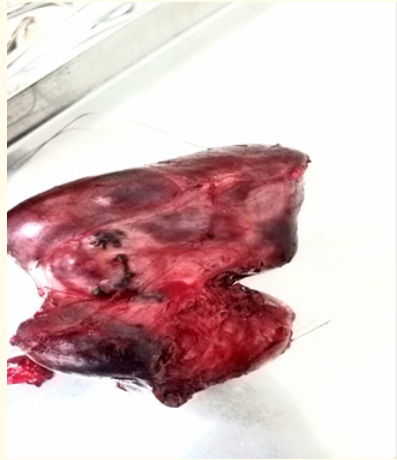
Figure 3: The specimen (right liver lobe).
The postoperative period was uneventful, so the patient left the hospital on the fifth post-operative day (POD).
The histological study showed HB with free resection margins (Figure 4).
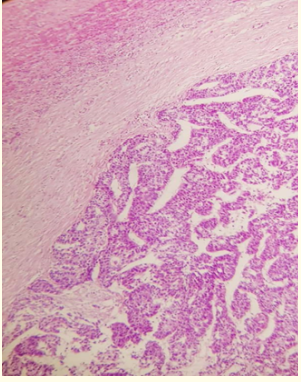
Figure 4: Micrograph showing a hepatoblastoma (right of image) and normal liver (left of image).
The follow up after four months showed no complications. No local recurrence or metastatic disease.
Relapses in HB are rare events occurring in less than 12% [9]. Increasing of the AFP level with hepatoblastomas during the onset of the disease is marked in 90% of cases [10] which was noticed in our case.
Complete resection for hepatoblastoma is the ultimate goal of treatment.
Although the best outcomes in the management of relapsed hepatoblastoma would happen after the combined treatment with chemotherapy and surgery [9].
The success of treatment for recurrent HB remains largely reliant on surgical resection. Surgical approaches vary depending on the location of the recurrent disease, when possible, complete resection of small lesions (intrahepatic, pulmonary, and cerebral) and/or liver transplantation for large localized lesions can be employed with curative intent.
Various chemotherapeutic regimens studied in small numbers of patients on phase I/II trials have shown few responses [11].
The best available data indicate that doxorubicin, if not given during initial treatment, and irinotecan are the most active agents in recurrent HB [11].
Our patient had chemotherapy without any response.
Complete resection of HB remains the golden choice of treatment.
The authors declare that there are no conflicts of interest regarding the publication of this paper.
The authors would like to thank Dr. Lina W.Assad for her help with histopathologic considerations and Dr Muhammad Ghanem for his great help in the editing.
Copyright: © 2020 Fadi Rayya and Turki Alhasoon. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.