Jean Mukwela1*, Gaby Kitambala2 and Dido Disengomoka3
1Pediatry, Hospital Complex of Kingasani II, Democratic Republic of the Congo
2Serologie and Biologie, Hospital complex of Kingasani II, Democratic Republic of the Congo
3Dated Manager, Hospital Complex of Kingasani II, Democratic Republic of the Congo
*Corresponding Author: Jean Mukwela, Pediatry, Hospital Complex of Kingasani II, Democratic Republic of the Congo.
Received: November 20, 2019; Published: December 10, 2019
Citation: Jean Mukwela., et al. “Study on the Genotypes Isolated from 2009-2017 in the Paediatric Hospital Complex of Kingasani in R.D.C”. Acta Scientific Paediatrics 3.1 (2020):11-14.
Three sites sentinels function since August 2009 in RDC, the Paediatric Hospital of Kalembe-lembe and the Paediatric Hospital complex of Kingasani II for the pool of Kinshasa and the hospital Jason de Sendwe with Lubumbashi.
The total of sample of the saddles collected by the Paediatric Hospital complex of Kingasani II is 1 615 cases including 891 positive cases with the rotavirus with a prévalence 55%.
What concludes that to safeguard the losses in human lives of our children from 0 to 59 months, it estnécessaire to introduce the vaccine into our country.
Keywords: Study; Genotypes; Insulated; Diarrhoea; Rotavirus; Paediatric; Prévalence; Infection
The infection with rotavirus is the principal cause of diseases diarrheal acute and serious, causing approximately 527 000 deaths per annum (WHO 2009) including 85% in the countries with low income as defined by the bank world (PATEL., et al. 2009).
IN 2003, the number of infectious episodes diarrheal causes annually by the rota virus in the world was regards with more than 111 million the cases; whose objective defined in our study is to determine the circulating genotypes and to introduce the vaccine.
We used as biological material: Saddles of the children suffering from gastroenteric during at least 7 days.
We employed an exploratory study based on the direct maintenance semi-structured with the parents of the patient children in a certain paediatric service of our country by identifying the elements hereafter:
The target of the monitoring for us within the framework of this study is all the sick children from 0 to 59 months victims of the acute diarrhoea requiring a hospitalization.
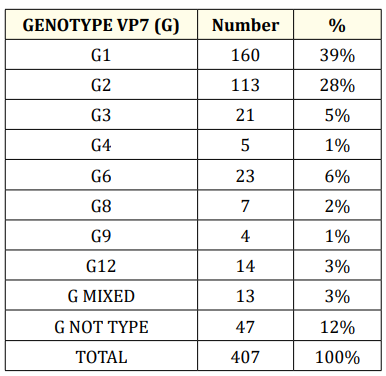
Table 1
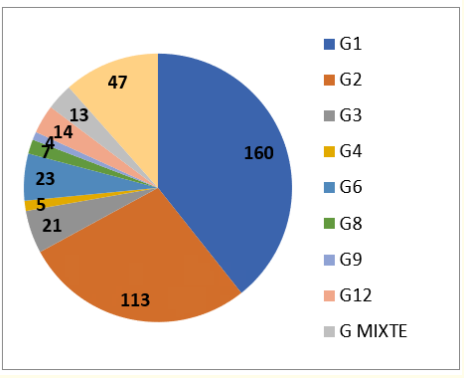
Figure 1
Genotypes G identified expresses that among the G genotypes, the most commonly found are G1 (39%), G2 (28%) and G6 (6%).
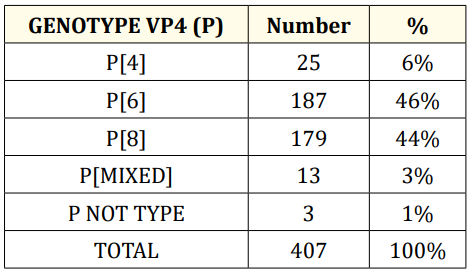
Table 2
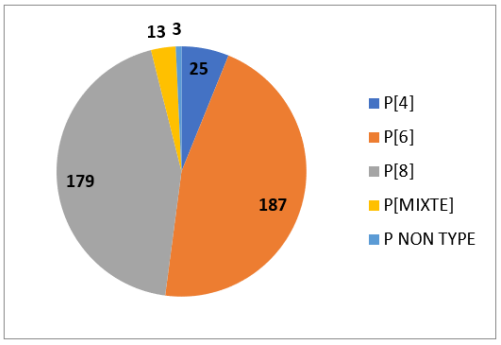
Figure 2
For genotypes P the table gives the following information: P genotypes, the most common are P [6] (46%), P [8] (44%) and P [4] (6%).
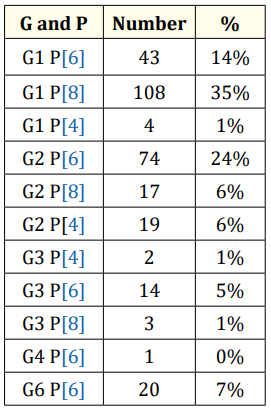
Table 3
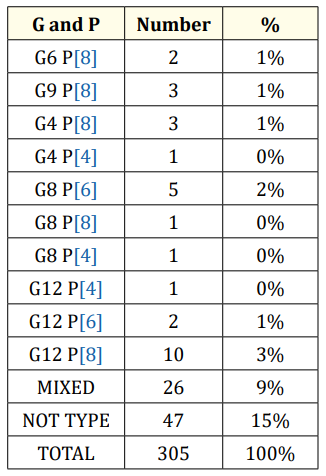
Table 4
To finish the genotype G and P combined, the combinations more prevail are:G1P[8](35%), G2P[6](24%) and G1P[6](14%).
The positive cases of the gastro entérites acute severe are most frequent in the children whose age varies from 0 to 24 months and the experiment shows us that the seasonal determination of the disease is carried out during the dry season (i.e. MAY, JUNE and JULY) which one records much case of the diarrhoeas with Rotavirus because it is at this time that the climate is cold in Kinshasa.
The resources and give them monitoring of this operational unit are managed by a coordinator on the level of the sites sentinel.
The laboratory of the site works in close cooperation with Biomedical the National laboratory of Research (INRB), the génotypage, the séquençage and the examinations of quality controls rest by the Regional laboratory of Reference, the WHO of the Mendusa university, Limpopo and the National laboratory of CDC Atlanta.
The PCR for the génotypage is carried out at the national laboratory of reference (LNR) of the INRB Kinshasa to (RDC), the regional laboratory of reference (RRL) of the university of Mendusa and to the national laboratory of Reference (CRL) of CDC Atlanta to the USA [1-9].
Our study proves that 55% of case of the children hospitalize for the severe diarrhoea are those whose age varies between 0 and 59 months that is allotted to an infection with rotavirus.
The results obtained in our study show and that it is necessary to introduce the vaccine into our country.
It is legitimate to wait until the effectiveness of vaccine anti rota virus in terms of protection is comparable with that of the infection does not protect from the reinfection, but protects nourrissons against the diseases severe with rota virus.
I thank the program widened for vaccination (PEV) to have placed at our disposal this study which made it possible in RDC to introduce into the monitoring system a new Anti-Rotavirus vaccine the 30/10/2019.
I thank WHO Afro, under office countries and the CDC to have to bring to the site sentinel the necessary means which allowed the correct operation of site.
I thank the Clelia Sister for his open spirit.
Our structure is with character social, works in the commune more populated Town of Kinshasa.We were obstinate fronts several difficulties, amongst other things financial which allowed rupture stocks (intra-reagent and other consumable) and misses motivation for the personnel of the site thus made, we are with the research of the financial partners for the continuter of the activities.
Copyright: © 2020 Jean Mukwela., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.