Miguel Martell1*, Marisa Burgueño2, Rafael Alonso3 and Tamara Herrera1
1Department of Neonatology, Facultad de Medicina, Udelar, Uruguay
2Department of Neuropediatrícs, Facultad de Medicina, Udelar, Uruguay
3Department Statistics, Facultad de Medicina, UDELAR, Uruguay
*Corresponding Author: Miguel Martell, Department of Neonatology, Facultad de Medicina, Udelar, Uruguay.
Received: October 10, 2019; Published: November 04, 2019
Citation: Miguel Martell., et al. “Impact of Neonatal Morbidities on Cognitive Outcomes of Very Low Birth Weight Infants at Childhood”. Acta Scientific Paediatrics 2.12 (2019):02-10.
Background: With the increasing survival of very immature infants, long-term neurological sequels remain high. Identification of infants at risk of special needs at childhood and implementation of early intervention strategies is poorly characterised.
Objectives: To evaluate the predictive value of the sum of major neonatal morbidities on cognitive outcome of surviving very low birth weight (VLBW) infants at school age.
Methods: A prospective cohort study including 92 preterm infants with a birth weight between 600 and 1500 grams born at a single tertiary center from 1998 to 2004. Data was collected at two-time points: neonatal hospitalization and school age. Primary outcome measures were (1) cognition evaluated at 8 years old by the Wechsler Scales of Intelligence for Children, third edition (WISC-III) and (2) school performance based on the need for special schools or grade repetition. The relationship between the number of morbidities and cognitive scale was studied with a multiple linear regression model. The effect of gender and social risk factors were also assessed. Results were compared with a control group (n = 18) of healthy full-term infants paired by their socioeconomic status.
Results: Most VBLW infants (70%) developed one or more morbidities. LOS was the most frequent complication (31%). Two infants died after discharge (2%); 71 surviving infants were available for follow-up at 8 years of age (79%). Fifty infants (70%) had a WISC III score ≥85; 17 (24%) had scores between 70 and 84, and 5 (7%) <70. Mean cognitive scores decreased progressively by 9 to 18 points with any additional neonatal morbidity (p<0.05), with the poorest cognitive outcome and need for rehabilitation programs for children with grade III or IV IVH and/or surgical NEC. Infants with 3 or 4 neonatal morbidities showed significantly greater neurological sequels at childhood compared to infants with 1 or 2 morbidities. Female gender and higher maternal education were independently associated with higher cognitive scores (p<0.05).
Conclusion: The sum of major neonatal morbidities provides an accurate measure of cognitive outcome at childhood for VLBW infants and thus can predict adequately the need for early intervention strategies and improve the quality of life of NICU survivors.
Gender and maternal education and socioeconomic status can also add to the prognosis.
Keywords: Major Neonatal Morbidities; Neurodevelopment; Childhood Outcomes; Cognition; Prematurity
NDI: Neurodevelopment Impairment; VLBW: Very Low Birth Weight Infants; BPD: Bronchopulmonary Dysplasia; NEC: Necrotizing Enterocolitis; LOS: Late-onset Sepsis; ROP: Retinopathy of Prematurity; IVH: Intraventricular Hemorrhage; PVL: Periventricular Leukomalacia; NICU: Neonatal Intensive Care Unit; WISC-III: Wechsler scales of Intelligence for Children, third edition.
Identifying neonatal morbidities that may impact on neurodevelopment in infants born prematurely plays an important role in clinical practice as well as identifying those infants at risk that may benefit from interventions at early stages and special, multidisciplinary follow-up programs [1]. Neurodevelopment impairment (NDI) is a significant long-term complication associated with preterm birth, and the risk increases with decreasing gestational age [2]. Improvements in perinatal and neonatal care has resulted in increased survival of extremely preterm infants (<28 weeks’ gestation) and many of them develop subtle limitations in later childhood such as learning disabilities, language difficulties, attention deficits, and behavioural disorders, to severe executive dysfunctions including loss of self-care that may result in a poor quality of life [3-6]. Increasing evidence shows that extremely preterm infants are at high risk of NDI at school age compared with term infants, mainly in cognitive aspects [7,8]. The relationship between neonatal morbidities such as bronchopulmonary dysplasia (BPD), sepsis, necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP) and severe intraventricular haemorrhage (IVH), and poor long-term neurodevelopment outcome has been thoroughly documented [9-11]. Recurring periods of hypoxia and acidosis, prolonged hospitalization and poor growth that adversely affect the developing nervous system are proposed mechanisms underlying this association [12,13]. Efforts have been focused in developing tools to quantify the impact of morbidities associated with prematurity in order to predict school age outcome in neonatal intensive care unit (NICU) survivors. Isolated major neonatal morbidities as well as neonatal risk scores composed by the sum of major medical complications and social risk factors have shown to predict overall cognitive ability and other neurodevelopmental outcomes in very low birth weight infants (VLBW, <1500 grams) [9,14]. Brain injury resulting from severe IVH and cystic periventricular leukomalacia (PVL) [9,15-18] as well as surgical NEC [19-22] are associated with the greatest risk of NDI and increased use of school services at childhood. However, the poor cognitive outcome of this vulnerable population is not only limited to medical complications but is also influenced by socioenvironmental risk factors such as socioeconomic status, home environment, family and parenting stress, and maternal education [5,14,23]. Gender-related differences have also been observed, as male infants have shown higher mortality and poorer long-term neurological outcome [24-26]. Predicting with accuracy the long-term impact of most common neonatal morbidities and other risk factors is needed to identify preterm infants at risk of NDI that will benefit from early intervention programs and educational support services. We studied the predictive value of the sum of major neonatal morbidities with and without inclusion of brain injury and surgical NEC on neurodevelopment outcome at school age of infants born prematurely with a birthweight between 600 and 1500 grams.
This prospective cohort study included 92 infants with a birthweight between 600 and 1500 grams (median 1075 g, SD 218) and a gestational age between 24 and 34 weeks’ (29 weeks’, SD 2.3) born at or admitted to a Uruguayan NICU from October 1998 to September 2004. Fifty-five percent of these infants were females. Data was collected at two-time points: neonatal hospitalization and school age. Infants were evaluated since birth and all neonatal morbidities with confirmed diagnosis during hospitalization were documented. Infants without a clear diagnosis were excluded from the study. Of the 92 infants selected for the study, 2 died during the first year of life due to respiratory complications (2%) and 18 were lost to follow-up before 5 years of age (20%). One infant was excluded due to leukaemia diagnosed at 4 years of age. As a result, 71 (79%) had available follow-up data at a mean age of 8.5 years of age (Figure 1). None of these infants developed traumatic or severe diseases that could affect neurodevelopment outcome. No significant differences in gestational age, birthweight and number of morbidities were found among those infants with available followup data at school age and those lost to follow-up. The study was approved by the ethical committee at the institution.
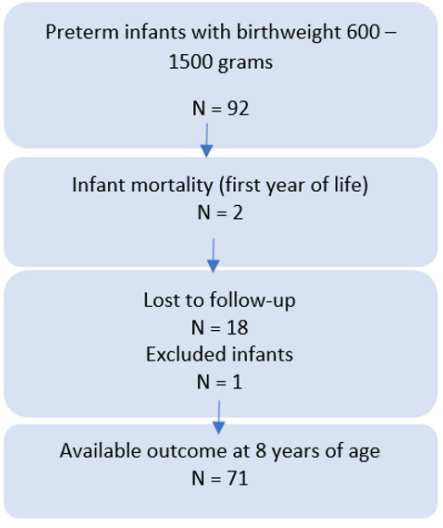
Figure 1: Flow of infants throughout the study.
Three groups were considered for the analysis of neonatal complications: (1) infants with no apparent morbidity (n = 21), (2) infants with one or more of the following morbidities: BPD, late-onset sepsis (LOS), severe ROP, apnea of prematurity, hyperbilirubinemia, hypoglycemia, hypernatremia, grade I or II IVH and medical NEC (n = 36), and (3) infants with any of the morbidities of group 2 who additionally developed brain injury and/or surgical NEC (n = 14).
BPD was defined as a need for supplemental oxygen at 36 weeks’ postmenstrual age (PMA) or when discharged home, whichever came first [27]. LOS was defined as blood or spinal fluid culturepositive infection that occurs after the first 3 postnatal days [28]. ROP was diagnosed by fundoscopic examination performed by a specialized ophthalmologist and was staged according to the international classification committee. Severe ROP was defined by the presence of stage 3 (extraretinal fibrovascular proliferation), stage 4 (partial retinal detachment) or stage 5 (total retinal detachment) [29]. Brain injury was defined as the presence of grade III or IV IVH and/or cystic PVL. Cranial ultrasonography was performed on all eligible infants at 3, 7 and 28 days of life. IVH was graded according to Papile’s classification [30]. Medical NEC was defined as stage II A or higher of Bell’s classification. Surgical NEC was diagnosed in infants requiring laparotomy or peritoneal drainage [31]. Other morbidities are defined in a previous study [32].
The main objective of this study was to evaluate the predictive value of the sum of major neonatal morbidities with and without inclusion of brain injury and surgical NEC on cognitive outcomes of VLBW infants at school age. Secondary outcomes included motor development and sensorineural functions. Data on growth, vision and audiometric characteristics were collected, and neurologic and developmental testing including assessment of cognitive ability and school performance were performed by trained physicians, nurses and psychologists in the follow-up visits.
Moderate to severe motor impairment was defined as total or partial movement limitation or abnormal muscular tone of one or more limbs. Hearing impairment was classified as moderate (hearing loss between 40 – 60 decibels) or severe (hearing loss between 61 – 80 dB) in both ears requiring amplification. During eye examination, visual acuity and extrinsic and intrinsic motility were assessed.
Cognitive outcomes were measured by (1) psychometric testing; and (2) school performance. Psychometric testing was performed through the Wechsler Scales of Intelligence for Children, third edition (WISC-III), an individually administered test of intelligence for the clinical and neuropsychological assessment of children between 6 and 16 years of age [33,34]. The WISC-III consists of ten subtests, each classified into a verbal or performance scale. The child’s performance on each subtest yields 3 composite scores: the verbal, the performance and the Full-Scale Intelligence Quotient (FSIQ). The scores on the verbal and performance subtests combine to yield the Full-Scale IQ score. The standardized mean of each score is 100 (SD = 15).
School performance was assessed based on the need for special schools for severely disabled children or any other form of special education in regular schools and/or the record of grade repetition. This information was obtained from the parents and confirmed by school records. Special education included physiotherapy, occupational therapy, phone audiology, and special classes. Socioeconomic data was collected from parents such as level of education and employment. Cognitive outcome was compared with a control group of 18 children between 7 and 8 years of age born at term, with birthweight appropriate for gestational age, without neonatal morbidities and whose mothers had 10 or more years of education.
Neurodevelopment (motor, sensorineural and cognitive) impairment is described for each group of infants in relation to the number of morbidities, gender and maternal educational level. Multiple linear regression analyses were used to estimate the effects of the number of neonatal morbidities, gender, birthweight, and maternal educational level and socioeconomic status (a score was generated between the two) on the Full-Scale IQ score at school age. Mann Whitney test and t student test were used for the comparison of quantitative variables, as appropriate. Comparison of qualitative variables between groups was performed with the x2 test. P values were considered significant at <0.05 for all statistical tests.
During the neonatal period, 50/71 (70.4%) infants developed one or more morbidities. Twenty infants (28%) presented only 1 morbidity, 10 infants (14%) developed 2 morbidities, 15 infants (21%) 3, 4 infants (6%) 4 and only 1 infant (1%) developed 5 morbidities. LOS was the most frequent neonatal morbidity (31%). The most common association was BPD and LOS, presenting in 18 cases. Neonatal morbidities and their frequencies are listed in table 1.
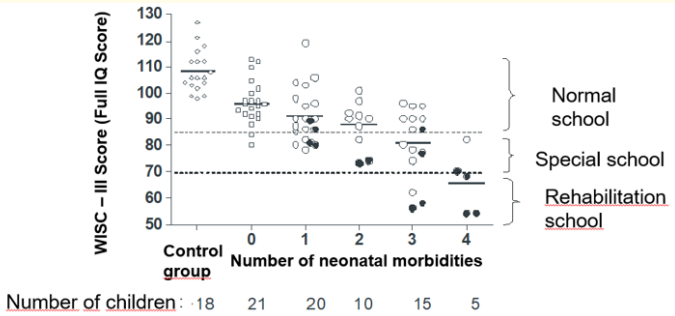
Table 1: Neonatal morbidities distribution (n = 50)*.
*Frequency was calculated based on the total number of neonatal
morbidities (106). Not all percentages sum 100 due to rounding.
**Described in methods.
Fifty infants (70%) had a Full-Scale IQ score of 85 or higher; 17 infants (24%) had scores between 70 and 84, and 5 infants (7%) less than 70. Mean Full-Scale IQ score for the control group was 107.4 (SE = 2.19); higher than groups 1, 2 or 3 for any morbidity (ANOVA, p<0.001). Mean Full-Scale IQ score for group 1 was 96.4 (SE = 1.85); significantly higher than group 2 (90, SE = 1.7) and group 3 (71.5, SE = 3.4). WISC-III score decreases significantly with increasing number of morbidities (p<0.05). The group of infants with brain injury and/or surgical NEC showed greater sensorineural sequelae and lower WISC-III scores when compared to those without brain injury and/or surgical NEC (Table 2).
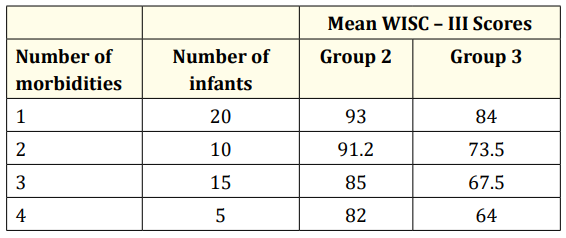
Table 2: Mean WISC – III Full IQ Scores by number of morbidities. Group 2: infants with 1 to 4 morbidities, without severe IVH and NEC; Group 3: infants with 1 to 4 morbidities, including severe IVH and/or NEC
Female infants showed higher WISC-III Full IQ scores compared to males in groups 1 and 2. Higher levels of maternal education (≥10 years) was associated with significantly higher WISC-III Full IQ scores in group 2. This association was not statistically significant for the rest of the groups (Table 3).
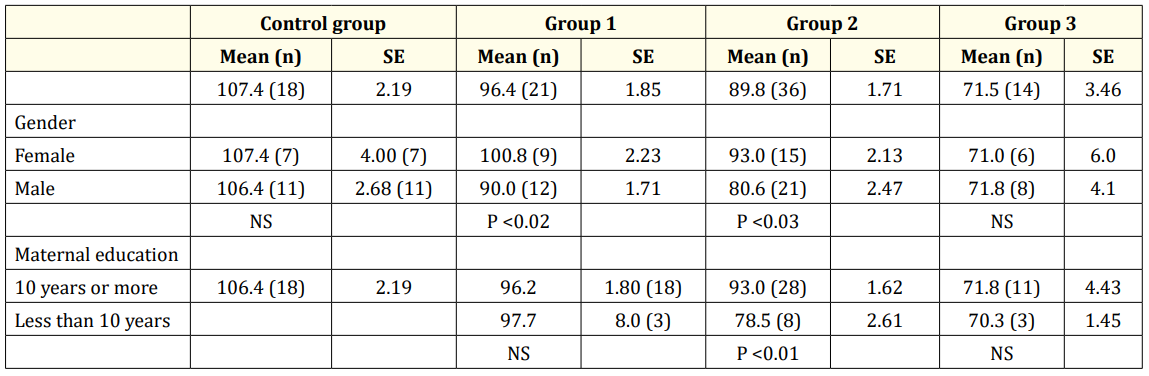
Table 3: WISC-III Full IQ Scores for the control full term group and preterm groups. Group 1: infants with no morbidities; Group 2: infants with 1 to 4 morbidities, without severe IVH and NEC; Group 3: infants with 1 to 4 morbidities, including severe IVH and/or NEC Abbreviations: SE; standard error, NS, not significant
Multiple linear regression analyses revealed that for group 1 of preterm infants the correlation between the number of additional neonatal morbidities (NAM -2.74, p = 0.001), female gender (7.24, p = 0.001) and maternal education less than 10 years (-9.75, p = 0.001), and NDI is statistically significant. For this group, the equation results as follows: Full IQ Score = 92.84 -2.74*NAM + 7.24.Gender -9.75*SES.
Constant = 92.84; NMA = number of additional morbidities; Gender: female = 1; male = 0;NSE = socio-economic level: low = 1; good = 0
However, the only statistically significant association for group 2 was the number of additional neonatal morbidities and NDI (NAM, -8.22, p = 0.002). Other variables, such as gender and maternal education, were not statistically significant. Therefore, the equation results as follows: Full IQ Score = 80.12 -8.22*NAM + 6.4.Gender. (80.12= Constant) Although not statistically significant, gender is included in the equation due to its biological importance. Table 4 summarises the above information, saves the calculations.
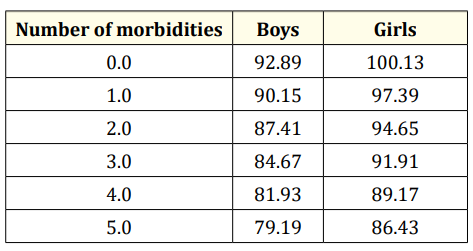
Table 4: Morbidities are able predict Full Scale IQ-Group 2. *If the economic status and maternal education are low 9.75 must be subtracted from the value of the table.
Fifty of the children (70% [95% CI: 60 – 80%].) with WISC Full IQ scores ≥ 85 attended regular schools. Forty of these children received additional education assistance during school years for variable periods of time, some of them permanently, especially those with speech and language difficulties. Children with Full IQ scores between 70 and 84 attended different types of schools, but all of them received some type of assistance. Those with Full IQ scores <70 required special education in schools with rehabilitation programs (Figure 2). Sixty percent of children (9/15) with scores between 70 and 84 repeated a grade level during the first 3 years of school, in contrast to an 8% (4/50) of children with scores ≥ 85 (OR = 17.25 [95% CI:17.2; 4.03 – 73.8].).
Diagram scatter of the full-term control group and the groups of preterm infants with and without morbidities in relation to WISC III Full IQ scores and school needs. This figure illustrates the mean cognitive outcome for children at 8 years of age with 1, 2, 3 or 4 neonatal morbidities compared to children without complications in the neonatal period. Mean scores were progressively lower and school needs progressively higher with any additional neonatal morbidity, with the poorest cognitive outcomes requiring rehabilitation programs at school age for those children with grade III or IV IVH and/or surgical NEC. Black dots correspond to infants with severe IVH and/or surgical NEC.

Figure 2: WISC – III.
The purpose of this study was to evaluate the impact of major neonatal morbidities on neurodevelopment outcomes of VLBW infants at school age. Results from this study show that the number of neonatal morbidities may predict cognitive outcomes at school age. Although brain injury and surgical NEC were the least prevalent morbidities during the neonatal period, these complications were associated with the lowest cognitive scores at childhood and need for rehabilitation schools. Sepsis and BPD were the most prevalent neonatal morbidities but were less predictive of poor cognitive outcomes and use of special schools. Multiple linear regression analyses showed the independent association between number of neonatal morbidities and cognitive outcomes at school age. Mean Full IQ scores at 8 years of age decreased progressively by 9 to 18 points and school needs were progressively higher with any additional neonatal morbidity. Significant differences were found between children with 3 or more neonatal morbidities and those with 1 or 2 (p<0.01). These findings are consistent with previous studies, which show more frequent and more severe neurological sequels in preterm infants with grade III or IV IVH [5,7,9,14,16,17]. and surgical NEC [19,21,22]. A significant reduction of 17 points in the WISC-III Full IQ Score has been previously described in children with 3 neonatal morbidities [9].
Cognition affected in a greater extent than motor function, as 31% of the preterm children of this cohort had a Full IQ Score <85 compared to an 11% rate of severe motor impairment. Periventricular white matter lesions and myelinated white matter volume loss followed by impaired cerebral cortical development are responsible for both motor and cognitive impairment in preterm infants [35,36]. In our study, IVH was identified in 9 surviving VLBW infants. Consistent with previous reports, the rate and severity of cognitive impairment varied according to the grade of IVH, with poorer cognitive functioning with increasing severity of IVH explained, in part, by the underlying ventricular dilation and parenchyma infarction [17]. However, controversy remains in relation to low grades (I to II) of IVH and neurodevelopment outcome [37]. Patra., et al. described lower mean cognitive scores and higher rates of NDI at 20 months’ adjusted age in ELBW infants with grades I-II IVH compared to those with normal cranial ultrasound [38]. Similarly, Vohr., et al. found that preterm adolescents with isolated grade II IVH had higher rates of learning disabilities, including cognitive and executive function deficits, compared with term controls [39]. However, in our cohort of VLBW infants, no significant difference was observed for low grades of IVH in relation to cognitive outcome.
Surgical NEC has been previously linked to significantly worse neurodevelopment outcome compared to either medically treated NEC or prematurity alone, most likely associated with greater severity of disease [21,22,40]. With advanced stages of the disease, the injured gut contributes to a systemic inflammatory response that, unmitigated, can affect the preterm brain [41]. In our study, preterm children with brain injury and/or surgical NEC showed greater sensorineural sequels, lower WISC-III scores, and need for rehabilitation programs when compared to those without these neonatal morbidities.
The most common association was BPD and sepsis. Consistent with previous findings, chronic lung disease was not predictive of neurologic or cognitive outcome unless associated to another neonatal morbidity (expect for severe IVH or surgical NEC) [16,23,42]. This could be explained by the use of surfactant and less aggressive ventilation strategies in our study. Similarly, we did not find sepsis to be predictive of long-term neurodevelopment outcomes.
Gender and maternal education were the second most significant variables in predicting cognitive outcomes at childhood. Female infants showed higher cognitive scores compared to males, consistent with previous findings supporting the higher vulnerability of male infants to develop NDI [25,43-45]. Kesler., et al. examined microstructural brain changes in a cohort of 29 preterm children at 12 years of age and found that preterm male children had significantly lower white and grey matter volumes compared to females. [45]. Skiöld., et al. described poorer cognitive and language outcomes at 30 months’ adjusted age for male preterm infants which correlated with differences in brain volumes on structural MRI at term equivalent age [25].
The impact of parenting and maternal education on long-term cognitive outcomes of VLBW infants has been well documented by several authors [5,23,46-48]. Higher levels of maternal education are significantly associated with higher cognitive scores and measures of intelligence in VLBW children [5]. Consistently, in our study, higher levels of maternal education (≥10 years) were associated with an increase in WISC-III Full IQ scores by 12 points (95% CI: 2,3-21) in preterm children from group 2. However, this association was not observed in children with severe IVH and/or surgical NEC during the neonatal period. Improved neurodevelopment outcomes in preterm infants born to mothers with high levels of education could be explained by genetic and environmental factors such as early mother-child interaction, safe and stimulating family environment, less parenting stress, access to quality healthcare services and early intervention programs or educational support.
Differences in cognitive outcomes between full-term and VLBW infants with morbility are consistent with previous studies [9,16]. Luu., et al. described a mean difference of 11.7 points in the Full IQ scale (95% IC:8.7-14.7) between preterm children without brain injury and term controls at 12 years of age [16]. Similarly, our cohort of full-term infants scored 10.6 points higher than VLBW infants without neonatal morbidities in the WISC-III Full IQ scale (105.3 vs 94.7). The absence of white matter injury on cranial ultrasound or conventional magnetic resonance imaging (MRI) does not exclude brain injury, as some subtle histological abnormalities can only be detected by diffusion tensor imaging (DTI) [49,50]. DTI abnormalities in the preterm animal brain reflect cellular changes such as astrocytosis and loss of oligodendrocytes that are characteristic of white matter injury and result in delayed myelination [51]. Therefore, preterm infants categorized as without brain injury on conventional neuroimaging may have subtle cerebral insults that could explain, in part, the poorer cognitive outcomes compared to term infants [16].
Cognitive assessment at 8 years of age seems to be relative stable, however, changes have been observed during this period. Improvement in Full IQ scores by almost 10 points have been documented in 45% of VLBW children at 96 months’ adjusted age, except for those with early-onset IVH and significant brain injury [5]. Therefore, early intervention programs and school support is needed to improve cognitive outcome in infants at risk, particularly in those born to mothers with lower levels of education.
Limitations of this study are the relative small sample size, changes in neonatal practice over the last two decades that may affect outcome data.
Categorization of neonatal morbidities, together with the MRI at 40 weeks’ corrected age, can predict adequately the risk of developing long-term neurocognitive sequels in a timely manner. Early identification of VLBW children at risk is useful for opportune intervention. Poor school performance and academic success is a concern for parents and educators, resulting in an increased demand for supportive educational services, particularly for VLBW children with severe IVH and/or surgical NEC requiring rehabilitation programs. Neurodevelopment outcome can be predicted individually based on medical and social risk factors, without requiring sophisticated techniques or high-cost interventions. In clinical settings, it will create a permanent search for new intervention strategies to increase survival without morbidity, improving the quality of care and the ability to prepare the families and future caregivers of these children with special needs.
None.
None.
3. Saigal S., et al. “Comprehensive assessment of the health status of extremely low birth weight children at eight years of age: comparison with a reference group”. The Journal of Pediatrics 125.3 (1994): 411-417.
Copyright: © 2019 Miguel Martell., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.