Mohammed J Aboud*
Consultant pediatric surgery, pediatric surgery unit, the Maternity and Child Teaching Hospital, Al Diwaniya, Iraq
*Corresponding Author: Mohammed J Aboud, Consultant pediatric surgery, pediatric surgery unit, the Maternity and Child Teaching Hospital, Al Diwaniya, Iraq.
Received: August 29, 2019; Published: September 20, 2019
Citation: Mohammed J Aboud. “A Gaint Oral Teratoid Tumor Combined Natal Teeth in Pediatrics: A Case Report”. Acta Scientific Paediatrics 2.10 (2019):96-100.
Teratomas are benign neoplasms composed of all the three germinal layers. They are composed of tissue elements foreign to the anatomic site in which it arises. Only approximate 1.6% of these tumors are found in the oral region. Pure address within the oral cavity and the tongue is very rare. We report a case of a giant oral teratoma combined natal teeth discovered at birth, which was managed with success and followed up for six months. A full-term male neonate was born by vaginal delivery with a congenital tumor protruding from the mouth, a large pedunculated mass, which prevented oral feeding. After 10 days of neonatal intensive care unit (NICU) admission, the mass was excised under general anesthesia from the hard palate and a local palatal flap repaired the mucosal defect. Histopathology confirmed teratoma. This case provides hope to all colleagues to improve the outcome through precise prenatal diagnosis, evaluation, and management roadmaps of potential cases of epignathus.
Keywords: Oral; Teratoid; Epiganthus; Natal; Pediatrics
Teratomas are benign neoplasms composed of all the three germinal layers. They are composed of tissue elements foreign to the anatomic site in which it arises. Around 80% are located in the ovaries and sacral lesions, 7% are seen in the head and neck region, only approximate 1.6% of these tumors are found in the oral region [1]. Pure address within the oral cavity and the tongue is very rare. Solely few numbers of cases are reported within the literature to date [2]. Epignathus is a highly developed congenital teratoid tumor in the oral cavity. Its incidence ranges from 1 in 35,000 to 1 in 200,000 live births and it usually affects the posterior nasopharynx, sphenoid or hard palate and has a slight female predominance [3]. This type of tumor may cause multiple malformations and sequelae in the patients, particularly in the case where the tumor mass is between the palatine processes before the sixth week of gestation and grows markedly from the seventh to a ninth week [4]. It may prevent fusion of the nasal septum with the bilateral palatine processes, which results in cleft palate formation. The tumor mass may result in morbidity and mortality due to their virtue of the location resulting in airway obstruction and respiratory distress [5]. Thus, the treatment of this tumor is complex and requires multiple interventions by a multidisciplinary team [6]. In 1950, authors introduced the terms ‘natal teeth’ for teeth present at birth and ‘neonatal teeth’ for teeth that erupt within the first 30 days of life. The incidence of natal and neonatal teeth has been submitted in multiple studies [7].
We report a case of a giant oral teratoma combined natal teeth discovered at birth, which was managed with success and followed up for six months.
Written informed consent was obtained from the patients' parent who participated and managed in this report for publication of both cases and any accompanying images. The scientific and health committee in our Health directorate office approved this publication.
A full-term male neonate was born by vaginal delivery at midwife, he is the product of a full-term pregnancy from a 19 - year-old prim gravida mother. The pregnancy and delivery were uncomplicated and the intrapartum ultrasound examinations were normal. At birth, the weight was 2.660 kg, with a congenital tumor protruding from the mouth, a large pedunculated mass, which prevented oral feeding. Surprisingly we noticed loose teeth in the lower front region of the jaw [Figure 1, A-C]. Over the following days, the mass was noticed to increase in size and by the 2nd day, the infant showed severe respiratory distress. He was referred to our hospital for consultation; a nasotracheal tube and an oral feeding tube were inserted initially. Oral clinical examination revealed a well-defined mass measuring 8×6×4 cm, protruding from the oral cavity without adhesion to the oral vestibule, obliterating the buccal vestibule. It was soft in consistency and readily bled on touch, originating from the hard palate. The patient did not have a cleft palate. He was unable to close his mouth; his tongue was swollen, protruding, and displaced toward the palate. The ventral surface of the tongue was edematous. No communication between the tumor and the intracranial cavity could be detected. We recommended outpatient ears, nose, and throat follow-up, as there were no accompanying symptoms. Initial laboratory findings revealed a white blood cell count of 12.300/mm3, hemoglobin of 13.4 g/dL, erythrocyte sedimentation rate was 15 mm/hr. Serum electrolyte and blood urea nitrogen values are normal. Liver function tests were normal. Serum alpha-fetoprotein (AFP) was highly elevated (5600 KIU/L), B-human chorionic gonadotropin (B-HCG) was normal. Head, neck and paranasal sinus computerized tomographic (CT)-scan native study demonstrated an oropharyngeal soft tissue tumor 16,6 mm X 14,6 mm with fat density extended from upper oral cavity, attached to the hard palate, more on the right side, the bone intact with no erosion, the pharynx and larynx with normal boundaries. Opacification of the right sinuses by the fluid density with opacification of 3 - 4 teeth in the lower front region of the jaw [figure 2, A-C]. The nasal septum deviated to the right with non-patent right osteo meatal complex with no intracranial extension. The pediatric dentist carried his opinion that there was severe mobility (Grade II) associated with these teeth. A danger of aspiration of these teeth existed. A decision to extract them immediately was made after the prophylactic administration of vitamin K. The procedure was carried out on 3rd day of admission under local anesthesia and careful curettage of the sockets was performed in an attempt to remove any odontogenic cellular remnants that might otherwise have been left in the extraction site. After 10 days of neonatal intensive care unit (NICU) admission, the mass was excised under general anesthesia from the hard palate and a local palatal flap repaired the mucosal defect. The histopathologic study revealed a mass composed of mature glial tissue, a wide range of cellular differentiation, a disorganized combination of mature adipose tissue, mucin secreting glands, tooth structure, skin annexes, skeletal muscle, and bone. No immature component was present. There was no evidence of malignancy. The diagnosis of teratoma was submitted [figure 3, A, B]. The postoperative course was uneventful. The nasotracheal and the gastric tube removed on the six post-operative days. Masseteric function and swallowing slowly improved over 2 - 3 weeks. The infant was discharged after 3 weeks, and he was symptom-free at 6 months follow-up.
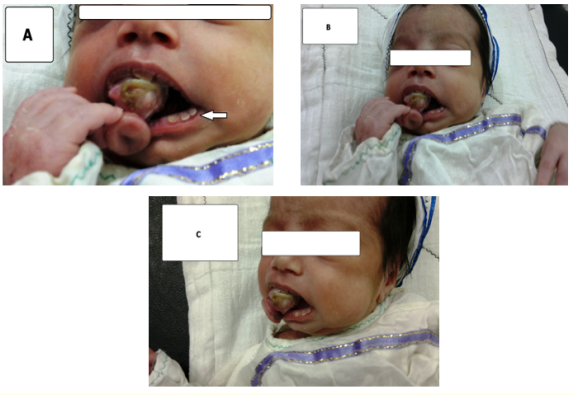
Figure 1 A-C: A well-defined mass protruding from the oral cavity without adhesion to the oral vestibule, obliterating the buccal vestibule with loose teeth in the lower front region of the jaw (arrowed).
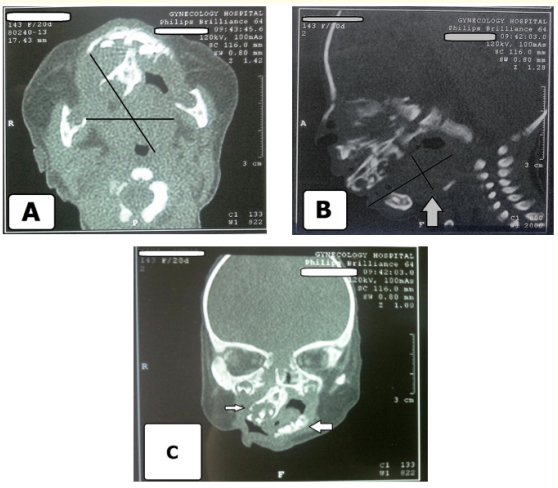
Figure 2 A-C: Native CT Images revealed A, oropharyngeal soft tissue tumor with fat density extended from the upper oral cavity, attached to the hard palate, more on the right side, the bone intact with no erosion, the pharynx and larynx with normal boundaries. B and C, opacification of the right sinuses by the fluid density with opacification of 3-4 teeth in the lower front region of the jaw (arrowed). The nasal septum deviated to the right (arrowed) with non-patent right osteo meatal complex with no intracranial extension.
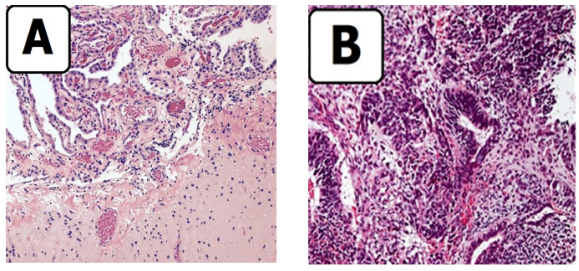
Figure 3 A,B: Histopathologic images revealed A, a mass composed of mature glial tissue, a wide range of cellular differentiation, a disorganized combination of mature adipose tissue, mucin secreting glands, tooth structure, skin adnexes, skeletal muscle, and bone. B, no immature component was present. There was no evidence of malignancy.
The term teratoma comes from the Greek word “teraton” (meaning monster) and designates tumors arising from pluripotent cells [8]. These tumors are composed of multiple tissues derived from the three germ cell layers (ectoderm, endoderm, and mesoderm). Oral teratoma is one of the most frequent sites for head and neck teratomas, and do not present a clear gender predilection and may arise from anywhere in the oronasal cavity [9,10]. In one study authors found malignant teratoma to be more common in men in a ratio of 5:4 [11]. Oral teratomas have been classified into four subgroups: first, dermoid or hairy polyps that are the most common and contain only epidermal and mesodermal elements. Second, teratoids that consist of ectoderm, mesoderm, and endodermal elements but that are incompletely organized. Third, true teratomas that contain all three germ layers but with the greater histological organization and recognizable early organ differentiation. Fourth epignathus that are highly differentiated tumors with well-formed organs and limbs, which are rare and generally incompatible with life [12,13]. Another pathologic variation of epigraphic is fetus-in-fetus, which may be considered incomplete twinning of monozygotic twins at a primitive stage when axial development begins [14]. The most common theory supposes that an epignathus derives from pluripotential cells in Rathke’s pouch that grow in a disorganized manner [15]. Many authors submitted that epignathi have been found to be associated with chromosomal abnormalities, such as duplication of 1q and 19p, ring X chromosome mosaicism, and the 49, XXXXY karyotype disorder [16,17]. Other studies have reported epignathi with no chromosomal abnormalities [18,19]. The parents in our case requested that we can not perform chromosome analysis, it was not available in our unit and too costly in the private sectors; accordingly, we were unable to establish a relationship between the tumor and the chromosomal assessments. Teratomas may be diagnosed antenatally by imaging, which permits early multidisciplinary management. In oral teratoma the most common symptoms are upper airway obstruction, dysphagia, and failure to gain weight [20]. Natal teeth are a distinctly rare condition defined as teeth present at birth, and neonatal teeth is which erupts within the first month of life [21]. The most common site of eruption is the lower central incisor area. The etiology of these teeth is still unknown, and careful evaluation of infants with natal or neonatal teeth is mandatory for possible syndromic conditions [22]. Our case was referred because of respiratory and feeding problems. Polyhydramnios and the severity of respiratory distress correlate with the size of the mass. Large oral teratoma typically emerges during the neonatal period in the head and neck is associated with high infant mortality rate in the absence of a well-prepared resuscitation team or meticulous delivery planning to secure the airway, as the majority of these teratomas are associated with obstruction of airway and difficulty in intubation [23]. The neonate’s prognosis worsens as the size of the tumor increases. In the immature teratomas, tissues might arrange from embryonic to mature, which are scattered haphazardly throughout the tumor, which differ from the orderly organoid arrangement seen in a mature teratoma. Teratomas in the neonates are mostly benign and consist of mature tissue components [24]. While teratomas with malignant potential occur in adults and contain immature tissues, with a high incidence of malignancy. Incomplete resection and presence of primitive neural tissue are associated with a malignant relapse [20,23]. We must consider other clinical differential diagnoses of neonatal oral mass include embryonic congenital rhabdomyosarcoma, retinoblastoma, nasal glioma, heterotopic thyroid, cystic lymphangioma of the oral or nasopharyngeal regions, and sphenoid meningoencephalocele [10]. Assessment by the CT scan and Magnetic resonance imaging (MRI) play a key role in differentiating neonatal nasopharyngeal teratomas from other causes of neonatal neck mass [25]. Perinatal management of oral teratomas includes delivery by cesarean section and either resection operation on placental support (OOPS) or an Ex Utero intrapartum treatment (EXIT) procedure with or without tracheostomy. In centers that are more sophisticated, in utero fetoscopy management of fetuses with oral teratomas may be feasible in selected cases [26]. Unfortunately, we have neither such facilities nor experience in our pediatric surgery unit yet. Postnatal management of oral teratomas involves surgical resection, which is often curative, without recurrences if the initial surgery is successful.
Our case was extremely rare but important findings will assist our pediatric surgeons, pediatricians, and pathologists in developing future management strategies when they are enrolled or confronted with such cases. This case provides hope to all colleagues to improve the outcome through precise prenatal diagnosis, evaluation, and management roadmaps of potential cases of epignathus.
The author expresses sincere gratitude to all the pediatric surgery unit staff, pediatric dental outpatient's clinic, and all the colleagues in the radiology unit at the Maternity and Child Teaching Hospital, Al Qadisiya, Iraq, for their assistance.
The authors declare that they have no competing interests.
Copyright: © 2019 Mohammed J Aboud. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.