Mehwish David*, Naheed Turi and Sarwat Jahan
Reproductive Physiology Laboratory, Quaid-I-Azam University, Islamabad, Pakistan
*Corresponding Author: Mehwish David, Reproductive Physiology Laboratory, Quaid-I-Azam University, Islamabad, Pakistan.
Received: June 19, 2019; Published: July 30, 2019
Citation: Mehwish David., et al. “Evaluation of Genotoxicity Caused by Heavy Metals Emitted from Brick Kiln among Children in Brick Kiln Industry”. Acta Scientific Paediatrics 2.8 (2019):66-69.
The study was designed to investigate the qualitative analysis of metal detection in blood serum and DNA damage among children working at brick kiln sites. A total of 232 blood samples were collected from different areas of Punjab, Pakistan. Analysis of heavy metals in blood was done by Atomic absorption spectroscopy, while DNA damage in white blood cells was perceived through single cell gel electrophoresis. Highly significant levels of zinc were perceived in children living in the vicinity of brick kiln areas, while concentrations of cadmium, chromium and nickel were also detected in quite high quantities. The data revealed that health risks and DNA damage are associated in children that are exposed to heavy metals emitted from brick kilns.
Keywords: Heavy Metals; Genotoxicity; Children; Comet Assay
Environmental pollution is a matter of global concern [1]. In developed countries, awareness and maintenance of healthy environment are subjects of concern. However, developing countries lack such attitude. With the increasing need for industrialization, the rate of environmental degradation due to pollution is also increasing on a daily basis [2]. Different study groups have reported that exposure to elevated levels of heavy metals such as lead, cadmium or chromium in brick kiln soil is found to induce neurological, hematological, gastrointestinal and immunological pathologies and even neoplasms, in which chief target is heme synthesis enzymes, thiol-containing antioxidants and enzymes [2-4]. Usually diesel is used for combustion in kilns for baking of bricks. The products and byproducts formed by complete/incomplete combustion of diesel are known to have genotoxic, cytotoxic, carcinogenic, free radical generating and DNA damaging effects [4]. Exposure to heat and radiation can cause genetic mutations, DNA strand breaks, DNA protein cross-links, radiation pneumonitis, fibrosis and diffuse alveolitis [4].
The present study aims to investigate the DNA damage potential of heavy metals emitted from brick kilns among children living at brick kiln sites, Rawat Pakistan.
For sampling coal clay brick kilns at Rawat, near Rawalpindi, Pakistan were selected and visited. The study pattern included personal interview and questionnaire filling, measurement of height and weight and collection of blood samples.
The present study was conducted at Reproductive Physiology Laboratory, Department of Animal Sciences, Faculty of Biological Sciences, Quaid i Azam University Islamabad. To study the toxic effects of brick kiln emitted pollutants on child health as well as on DNA damage, brick kilns from different regions of District Rawat, Punjab province were selected and sampling was done from June 2018 to June 2019. The approval to conduct this study was obtained from Ethical Committee of the Department of Animal Sciences, Quaid-i-Azam University Islamabad, Pakistan.
Different brick kiln sites from District Rawalpindi were selected for present study and will be given a named as site 1, 2 and 3 and so on respectively. The brick kilns will be selected on the basis of production capacity, functionality and nearness to communities and operated throughout the year.
A total number of age groups of brick kiln children (n= 232) living for varied years of exposure to brick kiln pollutants as well as not exposed from aged 7-17 years were considered.
After having consent forms signed by their guardians, blood samples were collected from venous blood in a 5ml syringe by registered female/male nurse. The blood obtained was then strained into the 5ml heparinized tube and stored at -4˚C for atomic absorption spectroscopy.
The method followed in this study was based on the method of Tripathy., et al. (1997) with some modifications.
This practice includes, embedding of single cell (spermatozoa) in agarose, lysing the cells and liberating out DNA by electrophoresis to form a comet tail cleaving the head of intact DNA. Intact and damaged DNA is pictured by fluorescence microscopy and computed by image analysis.
The first step was the assemblage of sperms. Right epididymis was crushed with forceps in phosphate buffer saline (PBS) in order to collect sperms and kept at 37oC for comet assay. The sperms were diluted in PBS (1 ml). The colour HUE of the pictures were changed to black and white to clearly visualize the DNA damage.
Microscope slides preparation layered with cells in agarose
The whole procedure was performed under low light in order to avoid induced damage to DNA.
Frosted microscopic slides were moderately heated on slide warmer, sheltered with 100µL of 1% regular melting point agarose (RMPA) prepared in distilled water at 40ºC and straightaway covered with a large (22 x 50mm) coverslip. The slides were kept in a chilled tray and left at 4ºC for at least 30 min to let the agarose to solidify. The coverslips were then removed and second layer of 85 µL was made on top of the first layer containing 20 µL of sperm suspension and 65µL of 1% low melting point agarose (LMPA) at 37ºC. This cell suspension creates a layer on top of the first agarose layer, covered with a coverslip and permitted to solidify.
The cells were then lysed by eliminating the coverslips and immersing the slides in a histology jar containing freshly made cold lysis buffer (pH =10.3). The lysis buffer had 2.5 M NaCl, 100 mM EDTA, 10 mMTris Base, (pH= 10.3), 1% (w/v) Triton X-100 (Dithiothreitol) (Villani., et al. 2012). Triton X-100 was added just afore commencing the lysis. EDTA was dissolve with the aid of NaOH pellets @ 0.2 g/ 10ml of solution. The slides were incubated with lysing solution for 24 hr at room temperature. After the incubation the slides were sweep away with distilled water three times at 20 min pauses by transferring the rack from one jar to another, to remove traces of salt and detergent. The jar was topped with aluminum foil to minimize light.
Slides were steadily placed in columns in the electrophoresis tray fronting towards anode containing a mixture of 1200 ml distilled water and 300 ml neutral electrophoresis buffer (54 g/L Tris base, 27.5 g/L boric acid, 0.5 M EDTA) at pH=8.0. Boric acid was dissolved at 45ºC while stirring. Slides were then equilibrated with electrophoresis buffer for 20 min. Electrophoresis was accomplished for 20 min at 25V (0.714 V/ cm). When electrophoresis was finished, the buffer was drained from the tank and the tray removed. Slides were covered with aluminum foil and air-dried overnight at 5°C.
The slides were rehydrated with distilled water for 60 min stained with Acridine orange (300-400 µL of 20µg/ml of distilled water) and analyzed under epifluorescent microscopy (400X, Nikon AFX-1 Optiphot) and digital images were taken for succeeding analyses/scoring with TRITEK software. Following sperm DNA comet parameters were recorded.
Tail length (TL, µm), Tail DNA (TDNA, %) and Tail moment (TM, µm)
The data was tabulated in the form of mean, standard deviation and standard error. Analysis of data was performed with unpaired t test, carried by a computer software Graph Pad Prism (version 5).
In current study, two groups were studied, mentioned as Control (n=98) and Exposed (n=134, those children working in brick kiln industry) with mean age (years) of 9.88 ± 0.85 and 12.97 ± 1.32 years.
Significant difference (p<0.05) was found in the concentration of Cd, Cr, Ni in exposed group as compared to control groups. The concentration of Zn was significantly high (p<0.01) in exposed group as compared to control group. Concentration of these heavy metals are shown in Table 1.
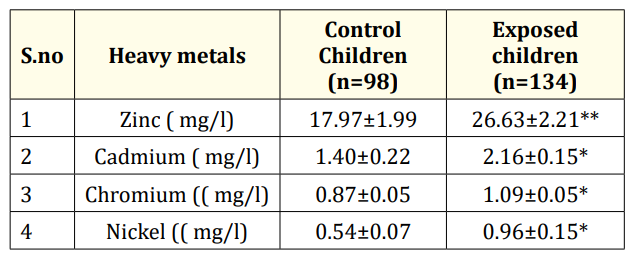
Table 1: Mean ± SEM heavy metals (zinc, cadmium, chromium,
nickel) concentration in whole blood of control group and exposed
group.
Values are expressed as mean ± SEM
*p< 0.05, **p< 0.01, ***p< 0.001
Figure 1 shows the DNA damage in exposed group as compared to the control group. A comet has been formed that shows the nuclear disposition and DNA damage as compared to control group.
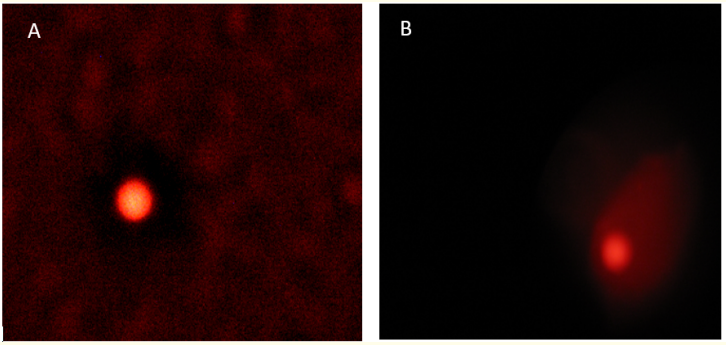
Figure 1: Diagram showing comet formed in control and exposed group. (a) The DNA is intact with no damage. (b) A comet tail has been formed which shows DNA displacement and DNA damage.
For centuries, air pollution has been considered to have detrimental effects on human health [5]. They cause large number of respiratory disorders such as chronic obstructive pulmonary disease, bronchitis, asthma, pneumoconiosis, emphysema, silicosis and asbestosis [5-8]. Considering different anthropogenic sources of pollution, soil is thought to be the most crucial source in developing countries due to the changes in the land use pattern during the past few decades [9]. As brick kiln industries are releasing poisonous gases as well as heavy metals that become part of the environment thus, imparting health risks among nearby populations. In current findings, the concentration of heavy metals i.e, cadmium, chromium and nickel was found quite high in blood drawn from children. Similar results have also been suggested by Jahan., et al. 2016 among male subjects [10].
Previously Kaushik., et al. [4] reported that exposure to heat and radiation can cause genetic mutations, DNA strand breaks, DNA protein cross-links, radiation pneumonitis, fibrosis and diffuse alveolitis [4]. Similarly, in present study, DNA damage with the formation of comet was evident in blood cells, that might be associated with genotoxicity induced by heavy metals in nucleus.
It is concluded from the present study that heavy metals get accumulated in the blood and become part of the circulation that may affect physiology of different body organs. Exposure to heavy metals induces genotoxic effects on blood in children living at/near brick kiln industries.
Copyright: © 2019 Mehwish David., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.