Kulvinder Kochar Kaur1*, Gautam Allahbadia2 and Mandeep Singh3
1Scientific Director, Dr Kulvinder Kaur Centre for Human Reproduction, Jalandhar, Punjab, India
2Scientific Director, Rotunda-A Centre for Human Reproduction, Mumbai, India
3Consultant Neurologist, Swami Satyanand Hospital, Jalandhar, Punjab, India
*Corresponding Author: Kulvinder Kochar Kaur, Scientific Director, Dr Kulvinder Kaur Centre for Human Reproduction, Jalandhar, Punjab, India.
Received: June 11, 2019; Published: July 10, 2019
Citation: Kulvinder Kochar Kaur., et al. “Optimizing Neonatal and Maternal Vaccination for Reducing Infant Morbidity and Mortality - A Short Communication”. Acta Scientific Paediatrics 2.8 (2019):31-36.
EPI got initially established in 1974 by WHO with the aim of giving universal immunization for children against diphtheria, pertussis, tetanus, poliomyelitis, measles and tuberculosis [1,2]. The Global Alliance for Vaccines and Immunization (GAVI) was established with a big coalition that included different UN agencies, and institutions (WHO, UNICEF, The World Bank), public health institutes, the vaccine industry and NGO’s so that the reach of EPI could be extended further by giving support to the poorest countries to ensure immunization for children under 1 year age, with the aim of achieving a world free of poliomyelitis, decreasing the incidence of tetanus and measles in developing countries along with introducing new and improved vaccines and technologies [3]. The WHO Summary of 2011 showed that the global coverage of DPT3vaccine (diphtheria, pertussis, tetanus) from 1980 to 2011increased from 20% to 83% and the Measles containinhg vaccines (MCV)shots from 16% to 85% [4].
The recommended immunization schedule in a country like Vietnam in 2015 is shown in table 1 [5] along with Indian recommended schedule in table 2.
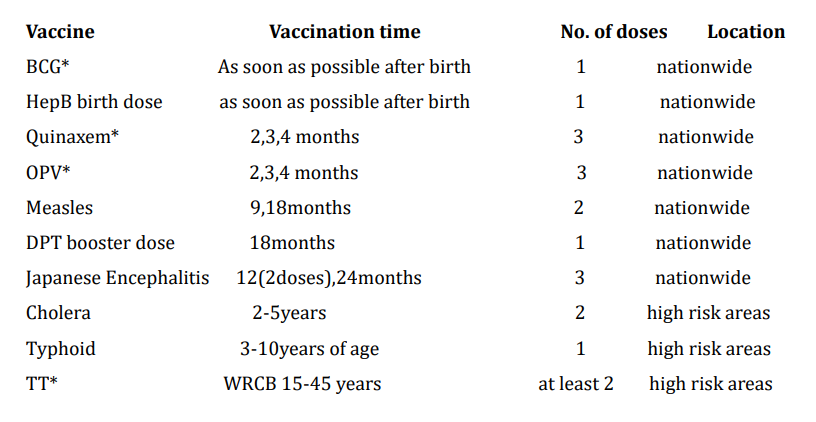
Table 1: Recommended national immunization schedule in 2015. *BCG-Tuberculosis vaccine, Quinvaxem (DPTHepBHib): diphtheria, pertussis, tetanus, hepatitis B, and Hemophilus influenza type b; OPV - Live oral Poliomyelitis; TT - Tetanus toxoid;WRCB15-45:women reaching childbearing age from 15 through 45 years of age(time that women are naturally able to become pregnant and give birth, or time that women have menstrual periods).
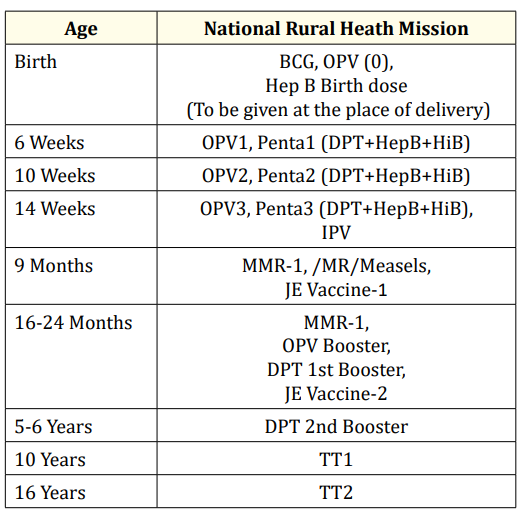
Table 2: Vaccination as per the National Immunization schedule by Government of India.
Bacillus Calmette-Guerin (BCG), one of the most widely used vaccines does not only protect against tuberculosis and other mycobacterial infections, but also has nonspecific (heterologous) immunomodulatory effects. Zimmermann., et al. conducted a randomized trial in participants, investigating the neonatal BCG immunization on antibody response to routine infant vaccines given in the first year of life. Antibodies against antigens in the diphtheria, pertussis, tetanus, polio, Haemophilus influenza type b (Hib) and the 13-valent pneumococcal conjugate vaccines were measured in 91 (45BCG –vaccinated, 46 BCG-naïve)infants one month after, and in 310 (169 BCG –Vaccinated, 141 BCG-naïve)infants seven mths after immunization at 6wks. 4 and 6mths of age. Additionally, antibodies against meningococcus C, Hib, measles mumps and rubella were measured in 147 (78BCG –vaccinated, 69 BCG-naïve infants one mth after immunization at 12mths of age. The seroprotect ion rate for each vaccine and the geometric mean concentrations (GMC) of antibodies were compared in BCG –vaccinated, BCG-naïve infants.
They found at 7mths of age seroprotect ion rates were high in both BCG –vaccinated, BCG-naïve infants. At 13mths of age seroprotection rates were lower than at 7mths of age, particularly of pertussis and a number of pneumococcal antigens, with generally higher rates for the latter in BCG –vaccinated infants. Although not statistically significant, antibody responses in BCG –vaccinated infants were consistently high against diphtheria, tetanus, and pneumococcal antigens age, at both 7and 13mths of age, and against measles at 13mths of age, but were lower against Hib one mth after immunization at both 7and 13mths of age. Thus concluding that the immunomodulatory effects of BCG on antibody responses to heterologous vaccines adds to the evidence that BCG immunization at birth has broad heterologous effects on the infant immune system [6].
Pertussis is an under –recognized cause of neonatal morbidity and mortality. Agarawal., et al. systematically reviewed information on the epidemiology and disease burden of neonatal pertussis in South and South Eastern Asian Countries, for which they used 3 bibliographic databases. Peer –reviewed original studies on neonatal pertussis epidemiology and disease burden published since 2000 with a geographic scope limited to South and South Eastern Asian Countries, were included. Data were systematically extracted based on parameters defined a priori. Their findings showed that the burden of neonatal pertussis and its complications is substantial. An increase in the number of pertussis cases has been noted since early 2000, ranging from61 to 92.9% in infants 0-3mths old. The most common symptoms an infant is likely tp present with are cough with or without paroxysms, cyanosis, apnea, tachypnea, difficulty in breathing and leukocytosis. Additionally it can =>hospitalization (length of stay; 5-7days), complications (pneumonia, seizures) and mortality ranging from 5.6 to 14.7%. Other observations indicate that the diagnosis is challenging because of nonspecific clinical symptoms. Specifically for obstetricians and gynaecologists, the information available for making informed decisions on the prevention of neonatal pertussis is unreliable. Maternal immunization against pertussis during late stages of pregnancy has proven to be efficacious and well tolerated. A high burden of neonatal pertussis, as well as its complications, is observed in South and South Eastern Asian Countries. Hence there is a need to intensify efforts to protect this vulnerable population with maternal vaccination [7a].
Measles remains one of the leading cause of child mortality worldwide and is now re-emerging in some countries due to poor vaccine coverage, concomitant with importation of measles virus (MV) from endemic areas. The lack of specific chemotherapies contributes to negative outcomes, especially in infants of immunodeficient individuals. Fusion inhibitor peptide derived from the MV fusion protein C terminal Heptad Repeat (HRC) targeting MV envelope fusion glycoproteins block infection at the stage of entry into host cells, thus preventing viral multiplication. To improve efficacy of such entry inhibitors Figuera Tiago., et al. modified a HRC peptide inhibitor by introducing properties of a self assembly into nanoparticles (NP)and higher affinity for both viral and cell membranes. Modification of the peptide consisted of covalent grafting with tocopherol to increase amphipathicity and lipophilicity (HRC5). One additional peptide inhibitor consisting of a peptide dimer grafting to tocopherol was also used (HRC6). Spectroscopic imaging, and simulation techniques were used to characterize the NP and explore the molecular basis of their antiviral efficacy. HRC5 forms micellar stable NP while HRC6 aggregates into amorphous, loose, unstable NP. Interpeptide cluster bridging governs NP assembly into dynamic metastable states. The results are consistent with the conclusion that the improved efficacy of HRC6 relative to HRC5 can be attributed to NP instability, which =>more extensive partition to target membranes and binding to viral target proteins [8] (figure 1).
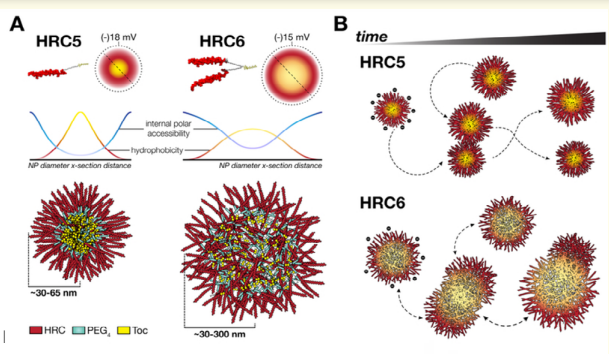
Figure
Other important vaccines are hepatitis B virus (HBV). Despite long term immune persistence observed following HBV vaccination of infants, the requirement of a booster dose following infant Immunization continues to be deliberated. Slowly C in 2018 gave evidence from HBV booster dose response studies and long term immunization program reviews are the basis for the recommendation that a vaccine booster is not necessary. However further studies continue to emerge and highlight the need for standardization among observational studies in order to appropriately compare outcomes. There is an assumption that neonatal and infant (within 12mths of age) vaccine immune responses being equivalent, however evidence is there that distinct vaccine immune responses are there within first year of life. HBV vaccine programs have evolved over time, especially regarding the type and dosage of vaccine used. Various universal neonatal immunization programs initially included a 2. 5µg dosage (Recombivax-HB, Merck). This dosage has been shown in multiple long term studies and meta-analysis to be associated with a lower primary response, reduced antibody persistence over time, and a decreased booster response 10-20 years following immunization. Ongoing surveillance of this and other HBV, neonatally vaccinated populations, particularly in low endemic regions, is essential to understand the impact on long term protection in order to ensure lifelong protection against hepatitis B virus Infection [9].
Two rotavirus vaccines, Rotarix 1 (RV1) and Rota Teq (RV5) were licensed for global use in 2006. A systematic review of 48 peer reviewed articles with post licensure data from 24 countries showed a median RV1 vaccine effectiveness (VE)of 84%, 75% and 57% in countries with low, medium and high child mortality respectively. A Partial vaccines series provided considerable protection, but not to the same level as a full series. VE tended to decline in the 2nd year of life, particularly in medium and high child mortality settings, and tended to be > against more severe rotavirus disease. Post licensure data from countries across geographic regions and with different child mortality rates show that under routine use both RV1 and RV5 are effective against rotavirus disease, supporting the WHO recommendation that all countries introduce rotavirus vaccine into their national immunization program [10].
Since the 1980’s, two lineages of influenza B viruses have been distinguished. These can cocirculate, limiting the protection provided by inactivated trivalent influenza vaccines (TIV’S). This prompted efforts to formulate quadrivalent influenza vaccines (QIV’s), to increase production against circulating influenza B viruses. Montomoli., et al. in 2018 described the results obtained from seven phase III clinical trials evaluating the immunogenicity, safety, and lot to lot consistency of a new quadrivalent split virion -influenza vaccine (Vaxigrip Tetra) formulated by adding a second B strain to the already licensed TIV. Since Vaxigrip Tetra was developed by means of a manufacturing process strictly related to that used for TIV, the data on safety profile of TIV are considered supportive of that of Vaxigrip Tetra. The safety and immunogenicity of Vaxigrip Tetra were similar to those of those of correspondingly licensed TIV. Moreover the new vaccine elicits a superior immune response toward the additional strain, without affecting immunogenicity towards the other three strains. Vaxigrip Tetra is well tolerated, has aroused no safe concerns, and is recommended for active immunization of individuals aged >6mths. In addition, preliminary data conform its immunogenicity and safety even in children aged 6-35mthas and its immunogenicity in older subjects (aged 66-80 years) [11].
Immunizing pregnant women is a promising strategy to decrease infectious disease related morbidity and mortality in pregnant women and their infants. Important prerequisites for the successful introduction of new vaccines for immunization in pregnancy include political commitment and adequate financial resources: trained, committed and sufficient numbers of healthcare workers to deliver the vaccines ;close integration of immunization programs with antenatal care by pregnant women in the country (especially in low and middle income countries (LMIC);and a high proportion of births occurring in health facilities (for ensuring maternal and neonatal follow up can be done). The framework needed to advance a vaccine program from product licensure to successful country level implementation includes establishing and organizing evidence for anticipated vaccine program impact, developing supportive policies, and translating policies into local action. International and national coordination efforts, proactive planning from conception to implementation of the programs (including countrycountry specific and cultural factors must be taken into account during the vaccine introduction [12].
Similarly another fact regarding high mortality from Bordetella pertussis occurs in hospitals where it is shown that the index case is usually an adult. In adults, the disease manifests mainly with persistent cough. Thus Navarrete., et al. conducted a study to determine the seroprevalence of Bordetella pertussis in the health personnel of a pediatric hospital in a country where vaccination of the staff is not considered mandatory. Nursing staff and resident doctors involved in direct treatment of hospitalized patients participated in the study. Each participant was screened for immunoglobulin G anti-pertussis toxin antibodies (anti –PT), and a questionnaire was applied for clinical and demographic data. 93 individuals were included, of which 85% were nurses, median age 35years (interquartile range: 29-42.5). The participants worked in the emergency department (21.5%), in the Pediatric intensive Care Unit (8.6%). and the Neonatal Intensive Care Unit (6.5%). Detectable levels of anti-PT antibodies were found in 18.3%, of which 53% presented titres suggestive of recent infection and only 23.5% cough >2week duration;Thus concluding that health personnel are at risk of suffering from the disease and can be potential transmitters to infants, who may die from this cause. This study suggested that the current vaccination policies in health personnel should be modified to determine the compulsory nature of the vaccination, especially in those individuals in charge of the care of the pediatric population [13].
Further Siasson., et al. carried out a systematic review for examining the timeliness of vaccination in preterm infants and to find any factors which were associated with timeliness. Since although vaccination is well tolerated and protective immune responses are observed, still some enquiries suggested that preterm infants experience unwarranted delays. The recent surge in pertussis cases and the increase in vaccinations administered are the cause of further exploration. They identified following a search of Medline, Cochrane databases of systematic reviews and the Cumulative index to nursing and Allied Health literature, where a narrative synthesis approach was carried out. 14 studies were identified, that indicated that infants with the lowest gestational ages and birth weights experienced the greatest delays. Vaccination timeliness was influenced by hospitalization and increased post discharge follow up. There was a lack of consensus to indicate that parental socioeconomic status and level of education were indicators for a delay. The studies proposed that many delays are unjustified and not according to genuine contraindications. Thus this review indicated that preterm infants are not vaccinated in a timely manner. Those involved in vaccinating preterm infants must be informed of the genuine contraindications. To avoid unnecessary delays putting preterm infants at an increased risk of infection. Hence care providers need to acknowledge the risk of delay in preterm infants and actively promote vaccination in this population. Regular training will help in regulating the inappropriate delays, and careful discharge planning is required to ensure that preterm infants are vaccinated on time [14].
The development of a group B streptococcus (GBS) vaccine for maternal immunization constitutes a global public health priority, to prevent GBS –associated early –life invasive disease, still birth, premature birth, maternal sepsis, adverse neuro developmental consequences, and to reduce perinatal antibiotic use. Sample size requirements for the conduct of a randomized placebo controlled trial to assess vaccine efficacy against the most relevant clinical endpoints, under conditions of appropriate ethical standards of care, constitute a specific obstacle on the pathway to vaccine availability. Alternatively, indirect evidence of protection based on immunologic data from vaccine and sero epidemiological studies, complemented by data from opsonophagocytotic in vitro assays sure, with subsequent confirmation of effectiveness against disease outcomes in post-licensure evaluations. Based on discussions initiated by WHO, Vekemans., et al. presented pivotal considerations about role of correlate of protection towards an accelerated pathway for GBS vaccine licensure and wide scale use. Priority activities to support progress to regulatory and policy decisions were discussed [15].
Another important factor is incomplete vaccination. Li., et al. retrospectively studied the data of 4525 children with special healthcare needs (CSHCN), who visited their clinic for a vaccination consultation from 1/1/2016 to may 30, 2018. Descriptive data were presented as mean + standard deviation (SD) and percentages. Multivariate analysis was done with non condition bivariate logistic regression to identify the underlying factors of vaccination recommendations. Subsequent information regarding the following vaccination and the occurrence of AEFI were also collected and analyzed. The main diseases consulted were those relating to the circulatory and nervous systems as well as neonatal diseases. The distribution of diseases varied by age: 53.6% infants under 12mths were counseled for circulatory system diseases, while 44.6% children aged 12-24 mths and 54.7% children >25mthswere counseled for nervous systems diseases. As per the evaluation reports issued by consultation clinic, 75% of CHCSN were recommended to be vaccinated normally, 21.2% were recommended to defer specific vaccination, while only 3.8% were recommended for all vaccinations. In logistic regression analysis, age, history of adverse events following immunization (AEFI) and the number of diseases combined were all strong corelative factors for vaccination recommendations. Children who were aged over 25mth old (OR=1.34.95% CI.1.11-1.61) or had a history of AEFI (OR=3.77.95% CI: 2.83-5.01) or those who had numerous diseases combined (OR=2.00, 95% CI: 1.46-2.75) tended to have greater rate of deferred vaccination recommendation. Among the CSHCN who received nationally recommended vaccines, the estimated AEFI rate was 24.29/100,000. No uncommon or rare serious adverse reactions were found. Thus concluding, age, history of AEFI and the no. of diseases combined were important factors that affected the vaccination recommendation of CSHCN. Most CSHCN can be safely vaccinated according to the nationally recommended vaccines schedules [16].
9855-9865.Oslowy C. “From-infanct and beyond----ensuring a life time of hepatitis B Virus (HBV) Vaccine induced immunity”. Human Vaccines and Immunotherapies8 (2018): 2093-2097.
Copyright: © 2019 Kulvinder Kochar Kaur., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.