Fadi Rayya1*, Zeina Aljarkas2 and Lian Aljarkas3
1Department of General Surgery, Al Assad University Hospital, Faculty of Medicine, Damascus University, Syria
2Department of General Surgery,Al Assad University Hospital, Faculty of Medicine, Damascus University, Syria
3Medical Information Officer, Damascus, Syria
*Corresponding Author: Fadi Rayya, Consultant Surgeon, Department of General Surgery, Al Assad University Hospital, Faculty of Medicine, Damascus University, Syria.
Received: February 22, 2019; Published: April 05, 2019
Citation: Fadi Rayya., et al. “Major Hepatectomy by Hepatoblastoma (HCV positive patient). A Case Report and Literature Review”. Acta Scientific Paediatrics 2.5 (2019):08-12.
Hepatoblastoma (HB) is considered to be one of the most common liver malignant tumors in children. An eight months old male presented with a huge hepatic mass, which was diagnosed as Hepatoblastoma. After neoadjuvant chemotherapy following the SIOPEL (the Childhood Liver Tumor Study Group of the International Society of Pediatric Oncology) (Société Internationale d’Oncologie Pédiatrique – Epithelial Liver Tumor Study Group) protocol, that did not make any improvement in down staging the tumor, nor did change the planned surgical approach (right hepatectomy). The patient underwent a successful right hepatectomy complication-less and he was discharged 3 days later
Keywords: Hepatoblastoma; HCV; Hepatoctomy; Syria
Hepatoblastoma (HB) is the most common primary liver neoplasm in children [1]. HB is generally diagnosed during the first three years of life. It is most commonly associated with constitutional abnormalities such as Beckwith-Wiedmann syndrome and familial adenomatous polyposis [2-4].
Premature births are also engaged with higher risk of developing (HB) as well as prolonged oxygen therapy of premature infants [5].
The age (6 months to three years old), increased Alpha Feto Protein level (AFP), and imaging findings suggested the diagnoses of (HB).
HB is found to be very responsive to chemotherapy, (agents as Cisplatin, Doxorubicin, Carboplatin, 5-Fluorouracil, Vincristine) and Usually down staging of tumors and control of the disease with pre-operative chemotherapy, leads to a less extensive liver resection, and the remission of metastasis [6,13,15].
An eight months old male, presented with abdominal mass with a good status, Abdominal exam revealed a palpable liver 3 cm beyond the right costal margin. Other than moderate anemia hemoglobin at patient had no comorbidities or known risk factors of HB.
Hematological studies showed moderate anemia (hemoglobin at 8.6 grams/dl), thrombocytosis (platelets 1.23200 x10⁹/L) it also revealed an elevated Gamma-Glutamyl transferase (GGT) (206) U/l (normal 0-30 U/l) and liver functions came ALT, AST (24) (92) U/l respectively. AFP was remarkably high with a value of >1000 ng/ml (normal value <20 ng/ml). The patient was also tested for HCV PCR and appeared to be positive.
Ultrasonography revealed a mass (5x6) cm in size, above and anterior to the right kidney located at the apex of the right liver.
Abdominal CT scan demonstrated a large mass occupying the right lobe of the liver, it is of uneven density nature (components with contrast enhancement, millimetric areas of necrosis, and focal calcification), close to the Inferior Vena Cava (IVC), no enlarged lymph nodes, no vascular invasion were detected (Figure 1).
An ultrasound guided biopsy was carried out, and it confirmed the diagnosis of (HB).
The patient, further then, was subjected to neoadjuvant chemotherapy according to the SIOPEL III protocol (doxorubicin- carboplatin). Patient received in all, 10 cycles prior to surgery in the course of seven months.

Figure 1: Abdominal CT shows the tumor and its relation to the IVC.
Afterwards, the patient underwent surgery, tumor was found very close to the IVC located in the V,VIII segments, a right hepatectomy was performed, and the tumor was completely resected without compromising the IVC, although, the tumor was adjacent to the IVC, it is also worth to mention that there was no need for blood transfusion during surgery (Figure 2-4), the postoperative period was uneventful, and the patient left the hospital on the third post-operative day in a good condition.
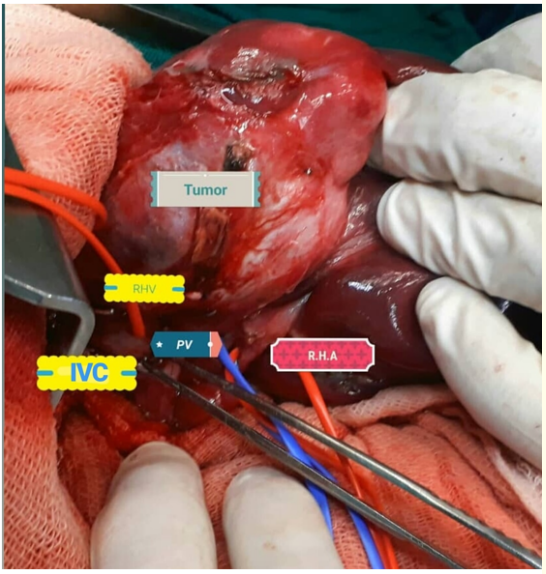
Figure 2: Intraoperative view.

Figure 3: Demarced right lobe.
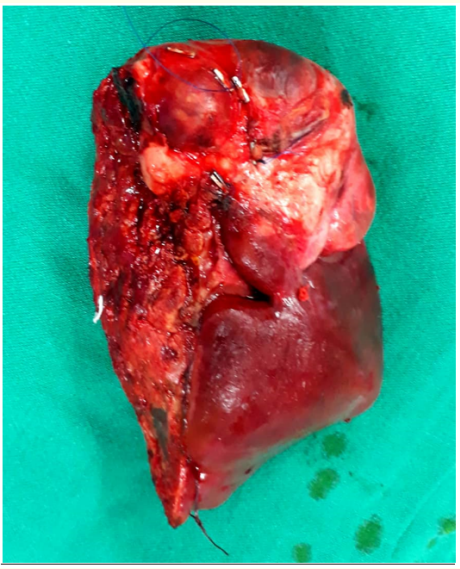
Figure 4: The speciment (right liver lobe Lsg V-Vlll).
Pathology showed Hepatoblastoma of Epithelial type, tumor measuring 5.5 CM at greater dimension, necrosis and hyaline degeneration with extra medullary hematopoiesis can be seen in 50% of tumor size (a response to chemotherapy). Capsular surface is uninvolved by invasive tumor. No lymphovascular invasion, the surgical margins were tumor free, Figure 5,6.
One-monthpost-surgery CT was performed and showed no trace of tumor. AFP came in still high with a value of 181 ng/ml. and the patient was readmitted to the hospital to take another two cycles of chemotherapy, which afterwards, the serum AFP measured (20.08 ng/ml).

Figure 5: Proliferation of round to polygonal cells smaller than the normal hepatocytes showing from cal rosettes formation with myxoid fibrosis.
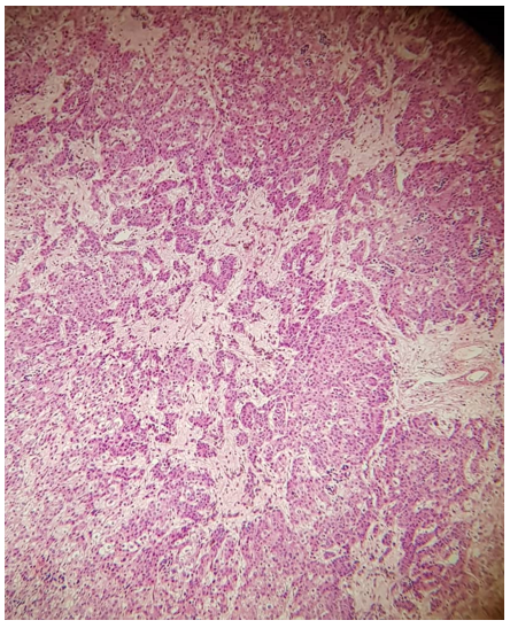
Figure 6: Post treatment effect with hyalinization.
Follow up studies up to ten months post-surgery revealed no recurrence of tumor.
Hepatoblastoma is a rare liver malignancy that is mostly seen in individuals younger than three years of age, although a few cases were reported in adolescence. With a happening rate of double in males than in females [8].
Our patient was suspected to have (HB) as he presented with an abdominal mass, elevated AFP, thrombocytosis, and the classic age to fit the diagnosis of eight months old (6 months to 3 years) [14].
Investigation of a suspected liver mass includes Ultrasonography, Computed Tomography, and Magnetic Resonance Imagining (MRI) to further investigate. MRI can sometimes be overlooked with a three-phase computed tomography which helps avoiding the sedation required to perform the MRI in these young ages [4], these imaging techniques performed on our patient reported a mass on the right lobe of the liver with components that increase the suspicion of HB such as (necrosis, calcification, and mixed areas of different contrast enhancement).
Laboratory investigations demonstrated a moderate anemia, thrombocytosis, and an incredibly high AFP level, along with elevated ALT, AST, and GGT. The patient was also tested for Hepatitis B and Hepatitis C, and he was positive for HCV.
Serum alpha-fetoprotein (AFP) is the most important clinical hematological marker for HBL, and remains the key clinical marker of malignant change, response to the treatment, and recurrence. However, there are some variants of both HBL and hepatocellular carcinoma (HCC) that have low or normal AFP level and usually come with a worse prognosis [9,10].
4 weeks post surgery AFP levels dropped to (181 ng/ml) and further more to (20.08 ng/ml) after 8 weeks from surgery. whilst, GGT and ALT rates remained high with a value of (300), (97) U/l respectively, and that elevation can be explained by the hepatitis C active infection (PCR).
Hepatic cell carcinoma (HCC) for a patient with HCV-related cirrhosis is approximately 2-6% per year [11]. And it is too a reason of elevated AFP in serum. By reviewing the literature, we have seen no relationship between the two conditions, although HCV is a very important factor in developing HCC, Figure a.
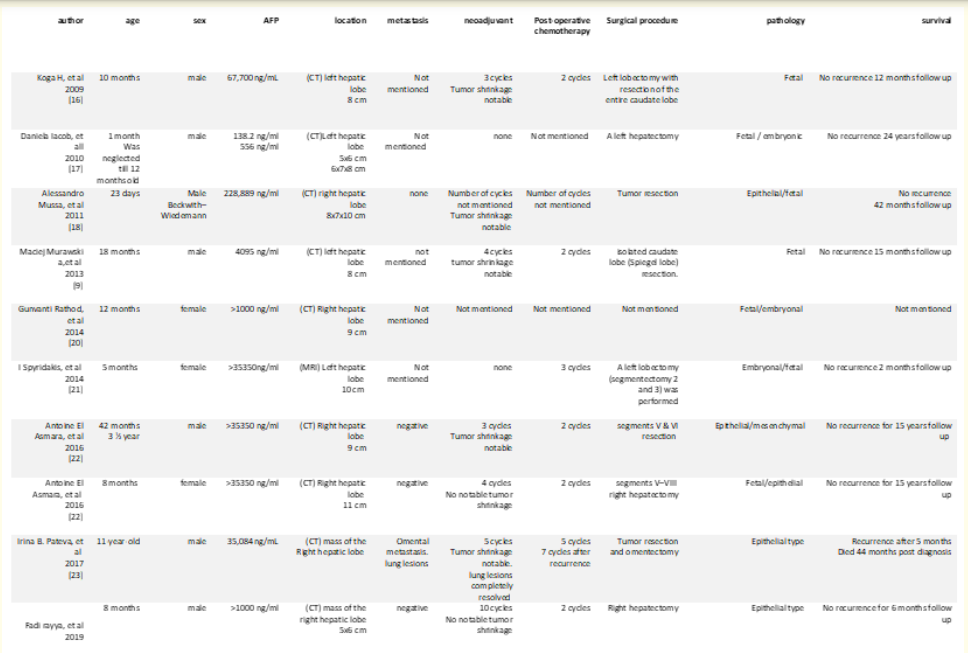
Figure a: Shows the litrutuer review.
Ultrasound guided biopsy is recommended by the SIOPEL, to confirm the diagnosis, as it hardly has any complications and was carried out on our patient and confirmed the diagnosis of Hepatoblastoma [7].
Preoperative chemotherapy usually leads to less unnecessary extended liver resection, as the recommendations are, to resect tumor in all, with high recurrence rate with incomplete resection [12].
In our case, even though, the platelets count normalized after the first course of chemotherapy still, our patient hardly experienced any tumor shrinkage after the neoadjuvant therapy, therefore, the surgical risk did not change (close tumor location to IVC).
While the neoadjuvant chemotherapy is highly recommended, researchers like the North American study groups, the Children’s Cancer Study Group (CCSG) and the Pediatric Oncology Group first, and the Intergroup Hepatoma Study, they promoted immediate surgery for localized tumors specially with the absence of metastases [4]. Because the surgery in our case is high risk, due to the location of the tumor we tried to lower that risk by applying neoadjuvant chemotherapy but it was with no remarkable benefits on the tumor size and its relationship to the IVC.
Surgery is considered as the curative therapy, if it is possible. In cases when the resection of the tumor is not possible, a total Hepatectomy and liver transplantation is advised [15-22].
Here we review some of the published cases of hepatoblastoma in the last ten years.
Most of the reported cases were younger than the age of one year old, and in which of them six were males to three females. Serum AFP levels were high at all cases reviewed. CT was the preferred diagnostic investigation in most cases. Nearly all patients were subjected to postoperative chemotherapy whilst, not all took neo-adjuvant chemotherapy. The most common pathological type was fetal-type hepatoblastoma either pure fetal, or mixed. The cases were monitored for different periods of time post operatively and the longest was fifteen years.
The reviewed medical cases have no records of such a case, and the prognosis of (HB) in an HCV positive patient, due to that, remains unknown, so we need further studies.
Chemotherapy is not always sufficient in down staging the initial tumor, the surgery remains the corner stone in dealing with HB. additional studies are required to produce more specified treatment regimens, and finally improve the overall outcome of both, chemotherapy, and surgical approach of (HB).
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient
No funding or grant support
All authors attest that they meet the current ICMJE criteria for Authorship.
The following authors have no financial disclosures: Fadi Rayya, Zeina Aljarkas and Lian Aljarkas
The authors would like to thank Dr. Lina W.Assad for her great help with histopathologic considerations.
Copyright: © 2019 Fadi Rayya., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.