Akbar Molaei*, Mahmood Samadi, Shamsi Ghaffari, Ahmad Jamei Khosroshahi and Ali Rafiei
Department of Pediatrics, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
*Corresponding Author: Akbar Molaei, Department of Pediatrics, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran.
Received: October 08, 2018; Published: November 22, 2018
Citation: Akbar Molaei., et al. “Early and Short Term Results of Transcatheter Arterial Duct Occlusion in Patients who had Pulmonary Hypertension with Congenital Cardiovascular Defects”. Acta Scientific Paediatrics 1.5 (2018):10-14.
Background: One important cause of children mortalities and morbidities is Pulmonary Hypertension (PH). A variety of congenital cardiac lesions can cause PH And it is vitally important to determine baseline hemodynamics and reaction to vasodilators in children with CHD and Pulmonary Vascular Diseases (PVD). It is still unclear to precisely determine hemodynamic values that correlate early and late outcomes in its best manner.
We present a case series study about early results of transcatheter arterial duct occlusion in patients with severe pulmonary artery hypertension associated by congenital cardiovascular defects.
Materials and Methods: The nine patients with congenital cardiovascular defects and PH were enrolled in this study. All of them underwent cardiac catheterization and transcatheter arterial duct occlusion by duct occlude devices. Hemodynamic study was done before procedure. Hemodynamic study was done before procedure. For cases with arterial blood oxygen saturation less than 95% or cases that have the ratio of Pulmonary Vascular Resistance (PVR) to Systemic Vascular Resistance (SVR) > 0.3 the hyperoxia test was done.
Results: 5 of the cases were female and 4 of them were male. The patients mean age and mean weight was 8.78 ± 6.50 (1 - 19) years and 23.53 ± 14.78 (6 - 24) kg respectively. The 3 of the patients were one year old and the 6 of them were older than 2 years. The defect type was patent arterial duct in four cases, patent arterial duct and atrial septal defect in two cases, patent arterial duct and ventricular septal defect in two cases and patent arterial duct and atrial/ventricular septal defects in one case. The hyperoxia test was positive and mean QP/QS increased from 2.15 ± 1.33 to 3.50 ± 1.73 and mean PVR/SVR decreased from o.49 ± 0.21 to 0.29 ± 0.12. The follow up period was 39 ± 18 months. The function class of the patients improved. The difference of the results was not meaningful between the patients younger and older than two years old.
Conclusion: The hyper oxia test is appropriate for evaluation of pulmonary vasoreactivity in the patients with CHDs and PH either younger or older than 2 years of age, but the cohort studies with more cases and long term follow up is necessary.
Keywords: Congenital Heart Defect; Pulmonary Hypertension; Arterial Duct Occlusion; Hyperoxia Test
One important cause of children mortalities and morbidities is Pulmonary Hypertension (PH) [1-3].
A variety of congenital cardiac lesions can cause PH. Patients who suffer from congenital cyanotic cardiac lesions such as high flow univentricular hearts, truncus arteriosus and great arteries transposition are more likely to develop rapid irreversible Pulmonary Vascular Disease (PVD). Those patients that suffer from PH and CHD are considered to compromise an inharmonious population. Different physiological issues appeared to contribute to occurrence rate and level of severity that vascular diseases emerge among patients that have CHDs. There is now convincing evidence that impaired production of NO appears at an early time point in patients with CHDs [4]. There is also evidence that suggest increased production of vasoconstrictor endothelin in patients who have PH and CHDs [5].
Many patients with high-flow CHDs that are operated upon in a timely fashion show a fall in pulmonary artery pressure and return to normal resting hemodynamics, indicating resolution and regression of pulmonary hypertensive structural changes.
However, some patients continue to have high levels of PVR and are persistent vasodilator therapy despite seemingly to be a mild vascular change (medial hypertrophy) on light microscopy. There are also others that in spite of on- time interventions and diagnosis develop progressive PVD in rapid manner. Prognosis, for these patients is not much beneficial than those who have indefinable PH [6].
It is vitally important to determine baseline hemodynamics and reaction to vasodilators in children with CHD and pulmonary vascular diseases. It is still unclear to precisely determine hemodynamic values that correlate early and late outcomes in its best manner. We present a case series study about severe pulmonary artery hypertension associated by congenital cardiovascular defects.
This study was done in madani heart center of Tabriz university of medical sciences at the northwest of iran. The patients with CHDs and PH were included which have patent ductus arteriosus (PDA) alone or accompanied by small atrial (ASD) or ventricular septal defect (VSD). All of them underwent cardiac catheterization and transcatheter arterial duct occlusion by duct occluder devices. Hemodynamic study was done before procedure. For cases with arterial blood oxygen saturation less than 95% or cases that have the ratio of Pulmonary Vascular Resistance (PVR) to Systemic Vascular Resistance (SVR) > 0.3 the hyperoxia test was done. The arterial oxygen pressure (PAO2 ), pulmonary to systemic blood flow (QP/QS) ratio, and PVR/SVR ratio were calculated before and after hyperoxia test. The changes of function class of the patients were recorded during follow up period. Data were analyzed by SPSS version 22 and kruskal-wallis and mann-whitney tests.
The nine patients with congenital CHDs and PH were enrolled in this study. 5 of the cases were female and 4 of them were male. The patients mean age and mean weight was 8.78 ± 6.50 (1-19) years and 23.53 ± 14.78 (6-24) kg respectively. The 3 of the patients were one year old and the 6 of them were older than 2 years. The defect types were PDA in four cases, PDA and ASD in two cases, PDA and VSD in two cases and PDA and ASD and VSD in one case. The hyperoxia test was positive for all the patients. mean QP/QS increased from 2.15 ± 1.33 to 3.50 ± 1.73 and mean PVR/SVR decreased from o.49 ± 0.21 to 0.29 ± 0.12. The follow up period was 39 ± 18 mo. The function class of the patients improved (Table 1). The difference of the results was not meaningful between the patients younger and older than two years old (Table 2).
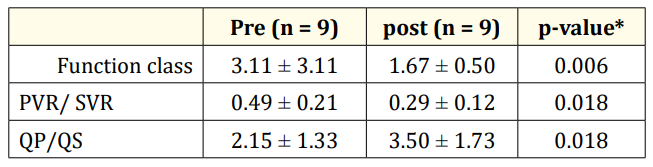
Table 1: the changes of the parameters before and after hyperoxia test.
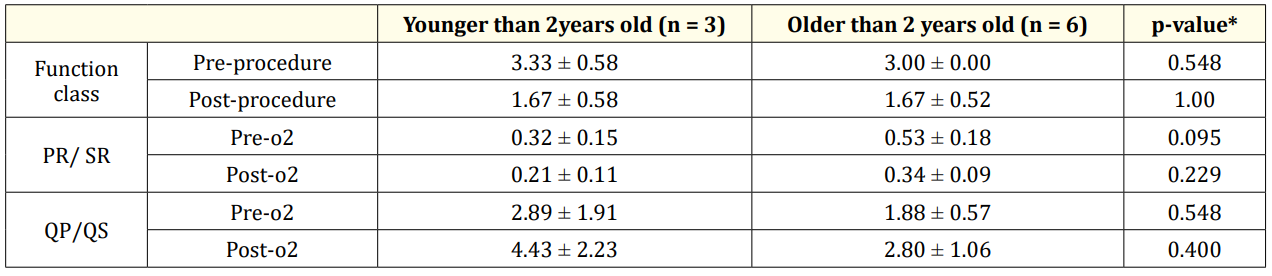
Table 2: comparison of the results between two age groups.
PH and PVD are the important cause of mortality and morbidity related to CHDs(1,2,3).The PVD can be evaluate by chest radiography [7,8], electrocardiography(ECG) [9], echocardiography [10- 16], MRI [17,18], Cardiopulmonary exercise testing or 6-minute walk testing [19-21], cardiac catheterization [22-24], wedge angiography [25], lung biopsy [26], morphometric analysis [27,28], intravascular ultrasound and optical coherence tomography [29], circulating level and activity of von-willebrand factor [30,31] and vasoreactivity tests.
It seems to be a complicate task to choose an appropriate method of PH therapy. This selection is suggested to be carried out carefully and must be based on etiology and determination of pulmonary vasoreactivity at cardiac catheterization and in general terms, VSD and Patent Ductus Arterious patients, before they are 2 years old, don’t develop irreversible pulmonary vascular changes.
In the absence of an appropriate surgical procedure it is estimated that about 50% of patients who have large nonrestrictive VSD will develop PVD [32].
It is vitally important to determine baseline hemodynamics and reaction to vasodilators in children with CHD and PVD for successful short- and long-term outcomes [33-36].
The age at which the surgery is done is the most important factor to have long-term survival and get free from PVD. Generally, having surgery on a CHD child before 2 years is recommended [3739]. It is while most medical centers do the same to completely repair the majority of lesions early in initial months of life.
In patients older than 2 years evaluation of the patients for PH and PVD before therapeutic intervention is necessary.
Cardiac catheterization aids in determination of PVR, PVR/SVR and QP/QS ratios. However, several issues arise in the determination of hemodynamics in these patients [40].
Combination of clinical, noninvasive, and invasive data should be used to make a decision about the possibility of operation in challenging children with shunt lesions and elevated PVR [41].
In this study our therapeutic intervention was according to clinical, noninvasive tests such as ECG, chest radiography and echocardiography and cardiac catheterization and vasoreactivity hyperoxia test.
Although there is inadequate long-term follow-up, use of vasoreactivity testing to determine operability suggests that those patients with a PVRI of 6 to 9 Wood units × m2 and PVR: SVR 0.3 to 0.5 may have a favorable outcome if there is a decrease in PVRI and PVR: SVR of at least 20% with a final PVRI of less than 6 Wood units × m2 and PVR: SVR 0.3 [42-44].
In this study the hyperoxia test was positive and the changes of PVR/SVR and QP/QS ratios were significant (table 1) patients with recurrent or persistent PAH after surgery have worse outcomes than those with Eisenmenger syndrome or unoperated PAH [45,46].
Our patients neither have persistent PH after hyperoxia test and duct occlusion nor recurrent PH during follow up period.
In this study the vasoreactivity to hyperoxia test was same for all the patients and there was not significant difference between the patients younger and older than 2 years of age.
Function class of the patients was improved after procedure and didn’t worsen during follow up period (table 2).
The hyper oxia test is appropriate for evaluation of pulmonary vasoreactivity in the patients with CHDs and PH either younger or older than 2 years of age, but the cohort studies with more cases and long term follow up is necessary.
Copyright: © 2018 Akbar Molaei., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ff
© 2024 Acta Scientific, All rights reserved.